Philips Consumer Lifestyle DL8765 Philips Wrist Blood pressure monitor with Bluetooth User Manual Leaflet A7 new branding 2015
Philips Consumer Lifestyle Philips Wrist Blood pressure monitor with Bluetooth Leaflet A7 new branding 2015
User Manual
Specifications are subject to change without notice.
© 2016 Koninklijke Philips N.V.
All rights reserved
Manufactured for:
Philips Consumer Lifestyle
A division of Philips Electronics North America Corporation
P.O. Box 10313, Stamford, CT 06904
4222.100.5748.1 (1/2016)
1
2
2
3
4
5
6
7
8
9
10
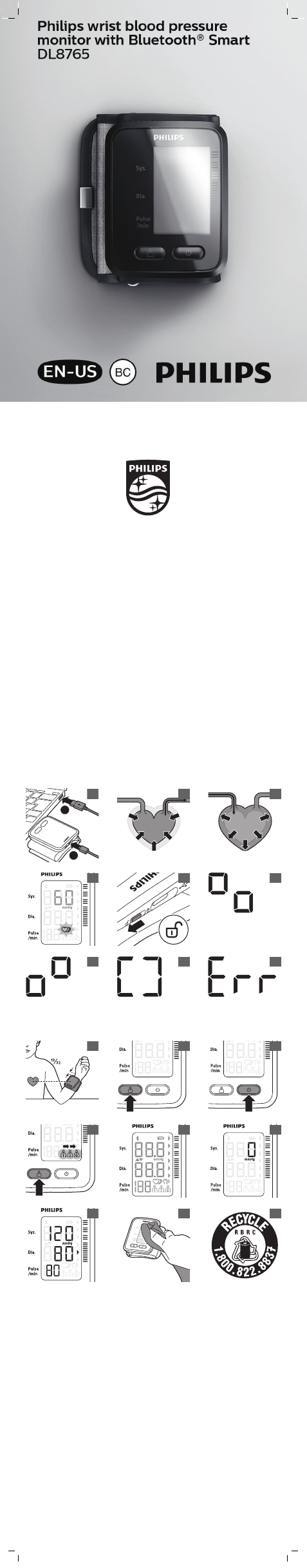
1 - 2 cm
"- ¾"
11
12
13
14
15
16
17
18
19
English
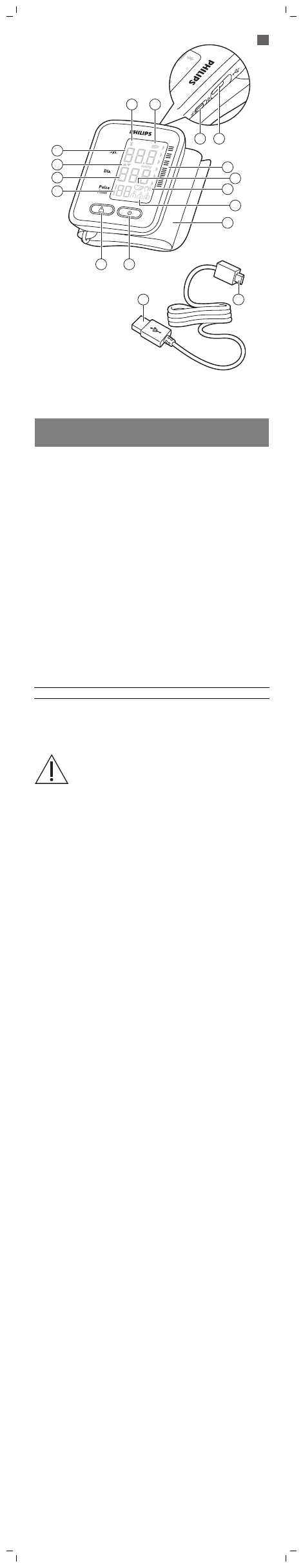
General description (Fig. 1)
1 Bluetooth® symbol
2 Battery symbol
3 Lock switch
4 Socket for micro USB plug
5 Blood pressure classification
6 Heart rate/irregular heart rate detector
7 Movement detector
8 User IDs
9 Cuff
10 On button
11 User ID button
12 Heart rate
13 Diastolic blood pressure
14 Inflation/deflation indicator
15 Systolic blood pressure
16 USB plug
17 Micro USB plug
IMPORTANT SAFEGUARDS
READ ALL INSTRUCTIONS BEFORE USING
When using electrical products, basic safety
precautions should always be followed, including
the following:
Warnings
- Please keep the unit out of reach of infants,
children or pets, since inhalation or swallowing
of small parts can be dangerous or even fatal.
- The device is not suitable for measuring the
blood pressure of children.
- No modifications of this equipment are allowed.
This may result in increased emissions or
decreased immunity of the device.
- This device is intended for non-invasive
measuring and monitoring of arterial blood
pressure. It is not intended for use on
extremities other than the wrist or for functions
other than obtaining a blood pressure
measurement.
- Do not use the blood pressure monitor during
charging as it can cause injury.
- Do not dispose of built-in batteries in fire.
Battery may explode or leak.
- Do not use while bathing and within 20 minutes
after taking a bath.
- The device is not suitable for persons who have
electrical implants.
- Do not reach for a corded device that has fallen
into water. Unplug immediately.
- A device should never be left unattended when
plugged in.
- The batteries used in this device may present a
fire or chemical burn hazard if mistreated. Do
not disassemble, heat above 100°C (212°F) or
incinerate.
- Do not use an extension cord with this device.
- Do not use this blood pressure monitor on any
arm where intravascular access or therapy
(such as an intravenous drip or a blood
transfusion), or an arterio-venous shunt (A-V
shunt) is present. The temporary interference to
blood flow by the blood pressure measurement
could result in injury.
- If you had a mastectomy (breast amputation)
do not use this blood pressure monitor on the
arm on the side of the mastectomy. The
inflating cuff can lead to pain, trauma and
further injury in the arm on the side of the
mastectomy.
- Consult your physician if you suffer from
illnesses prior to using the device.
- If you experience discomfort during a
measurement, such as pain in the arm or other
complaints, press the 'on' button to release the
air immediately from the cuff. Loosen the cuff
and remove it from your wrist.
- On the rare occasion of a fault causing the cuff
to remain fully inflated during measurement,
open the cuff immediately. Prolonged high
pressure (cuff pressure >300 mmHg or constant
pressure >15 mmHg for more than 3 minutes)
applied to the arm, may lead to bruises
(ecchymosis).
- Too frequent and consecutive measurements
could cause disturbances in blood circulation
and injuries.
- Beware of strangulation with the USB cord,
particularly for children and infants due to
cables.
12
5
7
9
1011
6
8
1 2
3 4
13
14
15
16 17
1

- This device is not intended for use outside a
home environment.
- Never use any accessories or parts from other
manufacturers or that Philips does not
specifically recommend. Using such accessories
or parts could cause a hazardous situation for
the user or damage to the device.
Caution
- Only use this device for its intended purpose as
described in this user manual.
- Always check the device and cuff before you
use it. Do not use the device or cuff if one of
them is damaged, as this may cause injury.
- The effectiveness of this blood pressure
monitor has not been established in pregnant
(including pre-eclamptic) women.
- Common arrhythmias (such as atrial or
ventricular premature beats or atrial fibrillation)
and peripheral artery disease / arteriosclerosis
can affect the performance (accuracy) of this
blood pressure monitor. Please consult your
physician how to best use this blood pressure
monitor if you suffer from any of these
conditions.
- Do not confuse self-monitoring with self-
diagnosis. This device allows you to monitor
your blood pressure. Do not begin or end
medical treatment based on the measurement
results. Always consult your physician for
treatment advice.
- Do not take any therapeutic measures on the
basis of a self-measurement. Never change
prescribed medication without consulting your
physician. Consult your physician if you have
any questions about your blood pressure.
- If you are taking medication, consult your
physician to determine the most appropriate
time to measure your blood pressure.
- This device is not intended for use on
extremities other than the wrist or for functions
other than obtaining a blood pressure
measurement.
- If the cuff pressure exceeds 300mmHg, the unit
will deflate automatically. If the cuff does not
deflate when pressures exceeds 300mmHg,
detach the cuff from the wrist and press the 'on'
button to stop inflation.
- Do not attach the cuff on the same arm on
which other monitoring medical electrical
equipment is attached simultaneously, because
this could cause temporary loss of function of
those simultaneously-used monitoring medical
electrical equipment.
- Never attach the cuff on injured skin, an injured
arm or an arm under medical treatment as this
can cause further injury.
- Do not use the device in case of existing
polyester or nylon material allergies.
- This device is not suitable for continuous
monitoring during medical emergencies or
operations.
- This device cannot be used with HF (High
Frequency) surgical equipment at the same
time.
- Only use the micro USB cable supplied to
charge the device.
- Avoid charging your blood pressure monitor in
extremely high or low temperatures (see
'Specifications').
- After charging, remove the micro USB plug from
the device and remove the USB plug from USB
portal.
- Do not attempt to replace your blood pressure
monitors battery. It is built-in and not
changeable.
- Keep the device away from fire and heat
sources, as the battery can overheat, causing
fire or bursting. The battery could explode,
causing injury or death.
- The equipment is not AP/APG equipment and
is not suitable for use in the presence of a
flammable anesthetic mixture with air, with
oxygen or nitrous oxide.
- To avoid measurement errors, do not use the
device near strong electromagnetic fields,
radiated interference signal or electrical fast
transient/burst signal. For example magnets,
radio transmitters, microwave ovens.
- Use this device under the right environmental
conditions as indicated in this user manual. If
not, this could affect the performance, lifetime
of the device and measurement results.
- This device is not washable. Never immerse the
device in water and do not rinse it under the
tap.
- Do not clean the blood pressure monitor when
it is being charged. Always unplug the charger
first before cleaning the blood pressure
monitor.
- If you have any problems with this device, such
as setting up, malfunction, maintaining or using,
visit www.philips.com/support or call
1-844-531-6861 for assistance.
- Do not open, disassemble or repair the device
yourself.
- Dispose of accessories, detachable parts, and
the ME equipment according to the local
guidelines.
Compliance with standards
- The device meets the relevant standards for this
type of Class II electrical medical equipment for
home use.
- This Philips device complies with all applicable
standards and regulations regarding exposure
to electromagnetic fields and complies with IEC
60601-1-2.
- This Philips device complies with applicable
standards and regulations of the FCC Rules.
SAVE THESE INSTRUCTIONS
Introduction
Congratulations on your purchase and welcome to
Philips! To fully benefit from the support that
Philips offers, register your product at
www.philips.com/welcome.
General
The Philips wrist blood pressure monitor with
Bluetooth® Smart enables you to perform blood
pressure measurements, heart rate (pulse)
measurements, transmit data via Bluetooth® Smart
to your mobile device and display your personal
measurement results in the Philips HealthSuite
health app. The device can also be used as a
standalone device.
This user manual contains important safety
information and provides step-by-step instructions
for using the blood pressure monitor.
Read this information carefully before you use the
blood pressure monitor and save it for future
reference.
Features
- 1 13/16" x 1 3/16" / 4.6 cm x 3.0 cm display with
white backlight
- Measure-during-inflation technology
- Supports 2 users
Intended use
The Philips wrist blood pressure monitor is a digital
monitor intended for measuring blood pressure
and heart rate in adult patient population, with a
wrist circumference ranging from 5 5/16 to 8 1/2" /
13.5 to 21.5 cm. The device is intended to be used
in an indoor home environment.
Display
Sym
bol
Description Explanation
Systolic
blood
pressure
Maximum blood pressure.
Also see section Systolic
and diastolic pressure
Diastolic
blood
pressure
Minimum blood pressure,
also see section Systolic
and diastolic pressure.
Heart rate Number of heartbeats per
minute (pulse is typically
equivalent to heart rate).
Battery
status
Indicates status of battery
during charging.
Measure
ment unit
Measurement unit of blood
pressure.
Inflation
indicator
The cuff is inflating.
Deflation
indicator
The cuff is deflating.
Irregular
heart rate
detector
Irregular heart rate
detection during the
measurement.
User IDs Start measurement for
selected user, and transmit
the measuring result.
Movement
detector
Moving during the
measurement will result in
an inaccurate result.
Blood
pressure
classification
Classification of measured
blood pressure following
WHO system (see 'Blood
pressure classification').
Bluetooth®
Smart
symbol
The device uses Bluetooth
for communication.
Heart rate
detection
Heart rate detection during
the measurement
Battery status indications
Battery
symbol
Battery status
The battery is almost empty.
+
The battery is empty.
When you measure 3 times a day starting with a
fully charged battery, the device can be used for
about 20 days until a recharge is needed. In case
of normal use, the battery can be charged around
300 times.
Note: Data will be lost when the battery is
completely empty.
Charging
The battery of this device is a built-in rechargeable
li-polymer battery with a capacity of 420 mAh.
Use the original USB cable supplied to charge the
battery.
When the battery is empty, it takes approx. 2 hours
to fully charge the battery of the device.
1 Put the micro USB plug in the socket of the
device (Fig. 2).
2 Put the USB cable in a USB port of a compatible
charger.
To ensure an optimal life time of the battery in the
product it is recommended to store it 50% charged
and re-charge every 3 months.
Battery charging indications
Battery
symbol
Battery charging indication
Battery charging: half full
Battery charging: almost full
Battery fully charged
Using the blood pressure monitor
This tubeless device uses the oscillometric method
to measure blood pressure and heart rate.
Before every measurement, the unit establishes a
“zero point” equivalent to the atmospheric
pressure. Then it starts inflating the cuff. During the
measurement, the device detects the pressure
oscillations in the blood vessels generated by the
heart pumping blood through the body. These
pressure oscillations are used to determine systolic
and diastolic blood pressure as well as heart rate.
While measuring heart rate, the device also
determines the small variations between the
individual heartbeats. If these variations exceed a
pre-defined threshold, the irregular heart rate
detector symbol lights up.
Systolic and diastolic pressure
The heart consists of two large chambers, the
ventricles and two smaller chambers, the atria. The
ventricles collect blood from the atria and expel it
towards the peripheral beds of blood vessels
within the body and the lungs. The atria collect
blood from these peripheral beds and prime the
ventricles.
When the ventricles contract and pump blood out
of the heart, the blood pressure reaches its
maximum value in the cycle, which is called
systolic pressure (Fig. 3).
When the ventricles relax and are filled again with
blood, the blood pressure reaches its minimum
value in the cycle, which is called diastolic pressure
(Fig. 4).
Blood pressure classification
Consult a physician in case of questions about
your blood pressure. Your physician can inform
you:
- About your normal blood pressure range.
- If your measuring result falls out of the range.
- Whether your blood pressure has reached a
dangerous level.
The following table shows the classification system
for the blood pressure measurements used in this
device. This system follows the classification
system of the World Health Organisation (WHO).
Blood pressure classification following WHO
system*
Systolic
pressure
mmHg
Diastolic
pressure
mmHg
Blood
pressure
indicator
³180 ³110 severe
hypertension
red
160 - 179 100 - 109 moderate
hypertension
orange
140 - 159 90 - 99 mild
hypertension
yellow
130 - 139 85 - 89 high to
normal
blood
pressure
green
120 - 129 80 - 84 normal
blood
pressure
green
< 120 < 80 optimal
blood
pressure
green
< 100 < 60 low blood
pressure
green
*Source: Chalmers J et al. WHO-ISH Hypertension
Guidelines Committee. 1999 World Health
Organization - International Society of
Hypertension Guidelines for the Management of
Hypertension. J Hypertens, 1999, 17:151-185.
Irregular heart rate detector
The device is equipped with an irregular heart rate
detector. An irregular heart rate is detected when
the heart rhythm varies above a pre-defined level
while the device is measuring the systolic and
diastolic blood pressure. During each
measurement, this device records the heartbeat
intervals and calculates the standard deviation. If
the standard deviation exceeds a pre-defined
threshold, the irregular heart rate detector symbol
lights up when the measurement results are
displayed (Fig. 5).
Caution:The appearance of the irregular heart rate
detector symbol indicates that a heart rate
irregularity was detected during measurement.
Usually this is not a cause for concern. Due to the
irregularity in your heart rate the blood pressure
measurement might not be accurate, i.e. it might
not reflect the 'real' situation in your body.
However, if the symbol appears often, we
recommend that you seek medical advice. Please
note that the device does not replace a cardiac
examination.
Preparing for use
Pairing the blood pressure monitor to
your Bluetooth device
Note: Before you use the device for the first time,
remove the protective foil from the display.
Note: Before using the device, make sure the lock
switch is placed in the ‘off’ position (Fig. 6).
Note: To switch on the device for the first time,
press the 'on' button for 3 seconds.
The blood pressure monitor is equipped with
Bluetooth® Smart. You can receive your personal
health data on a mobile device that is equipped
with the Bluetooth® Smart function. Download the
Philips HealthSuite health app from the App store
or Google Play. Use the search term 'Philips
HealthSuite health app'. The app is available for
iOS® 8.0+ and AndroidTM 4.4+.
Note: You can only use the Philips HealthSuite
health app to communicate with the device. It is
not possible to use third party applications.
1 Download the Philips HealthSuite health app
on your mobile device, start the app and follow
the instructions to create a user profile and add
the blood pressure monitor device.
2 Make sure the app is active and Bluetooth is on
when pairing is in progress.
-Keep the mobile device and the blood
pressure monitor within transmission range
(no more than 16 feet (5 meters) from each
other, in the same room).
3 With the device turned off, press the 'on' button
for 3 seconds, until it turns on in pairing mode.
-These symbols are shown on the display
alternately, indicating that the connection is
being established: (Fig. 7) and (Fig. 8).
4 When pairing is successful, the app shows this
symbol: (Fig. 9). The app shows which user
profile is assigned to you.
-If the connection fails, the display shows this
symbol: (Fig. 10).
-The blood pressure monitor has 2 user
profiles. If both user profiles are in use,
choose an existing profile to overwrite.
-You can also delete both user profiles by
pressing and holding the user ID button for
approx. 10 seconds. The display of the
device shows 'del'. All stored date is deleted
and you have to follow step 1-4 to pair and
add a new user.
5 The blood pressure monitor shows the
Bluetooth icon on the display as soon the
connection has been established and switches
off automatically after a few seconds.
When the blood pressure monitor is successfully
paired with your mobile device, the blood pressure
monitor automatically transmits your personal
health data to your mobile device via Bluetooth
Smart.
Note: Only when the Philips HealthSuite health
app is active, your personal health data can be
transmitted.
Measuring blood pressure
Tips for proper measurement
- Rest for 5 minutes before you measure your
blood pressure.
- Wait at least 3 minutes between measurements.
This allows your blood circulation to recover.
- For a meaningful comparison, try to measure
under similar conditions. For example, take
daily measurements at approximately the same
time, on the same arm, or as directed by your
physician.
- For a good Bluetooth® connection between the
blood pressure monitor and your mobile
device, make sure the two are close and there
are no obstacles between the two devices. We
recommend not to have the two devices farther
than 16 feet (5 meters) apart.
We advise you not to take a measurement under
the following circumstances, as this measurement
may not be representative:
- Within 1 hour after eating or drinking
- Immediately after smoking
- While bathing and within 20 minutes after
taking a bath.
- While you are talking or moving your arm, hand
or fingers
- In a very cold environment
- When you need to urinate
Attaching the cuff
1 Remove all jewelry, such as watches and
bracelets from your left arm.
Note: If your physician has diagnosed you with
poor circulation in your left arm, use your right
arm.
2 Roll or push up your sleeve to expose the skin.
Make sure your sleeve is not too tight.
3 Hold your arm with your palm facing up and
slide the cuff onto your left wrist.
4 Position the lower edge of the cuff 13/32" / 1 cm
above the palm of your hand (Fig. 11).
5 Fasten the cuff around your wrist, leaving no
extra room between the cuff and your skin. If
the cuff is too loose, the measurement will not
be accurate.
-The cuff will not cause any potential
sensitization or irritation of the skin. The
materials of the cuff have been tested and
found to comply with requirements of ISO
10993-5:2009, ISO 10993-1:2009 and ISO
10993-10:2010.
6 Correct posture for measurement:
-Make sure you do not wear tight clothing
during measurement.
-Sit comfortably with legs uncrossed, feet flat
on the floor. Make sure that you sit upright
with your back straight.
-Hold your arm up so that the center of the
cuff is at the same level as the heart to
ensure correct measurements (Fig. 11).
-Relax your wrist and hand. Do not bend your
wrist back, clench your fist, or bend your
wrist forward.
Start measurement
1 Before using the device, make sure the lock
switch is placed in the 'off' position (Fig. 6).
2 Press the user ID button (Fig. 12) or 'on' button
(Fig. 13) once, to switch on the device. The
device automatically selects the previous user.
-To change the user profile, press the user ID
button and select a different user (Fig. 14).
Make sure the correct user is selected, so the
measurement data is properly transmitted
and stored. It is not possible to switch a user
profile after a measurement.
-When the health app is open, the app
automatically selects the correct user profile.
In this case, the user profile can be changed
by either closing the app and reopening it
again with the correct user profile, or by
closing the app and using the user ID button.
-Also a guest user can be selected. A guest
user is a user without a user profile in the
app. The guest user is for performing a
measurement on other persons without a
user profile in the app. Measurements
performed when using the guest user are
not stored in the memory nor transmitted to
the app.
3 Attach the cuff to your wrist (see 'Attaching the
cuff') and make sure your posture is correct (see
'Tips for proper measurement').

4 Press the 'on' button to start the measurement
(Fig. 13). All display characters are briefly shown
on the display (Fig. 15). The device is ready for
measurement and the number 0 appears (Fig.
16). Inflation of the cuff starts automatically
which is indicated by the inflation indication
(see 'Display').
Note: If you experience discomfort during a
measurement, such as pain in the arm or other
complaints, press the 'on' button to release the
air immediately from the cuff. Loosen the cuff
and remove it from your wrist.
-During inflation, the unit determines the
systolic pressure and diastolic pressure as
well as heart rate. This is shown by the heart
rate detection symbol.
-The movement detector will light up when
movement is detected. This may result in
inaccurate measurement results.
5 When the measurement is finalized, the cuff
deflates and the measurement results are
shown on the display (Fig. 17). To transmit the
measurement results to the app, see section
'Transmit and store personal health data in the
app'.
6 Press the 'on' button to switch off the device.
Note: after 1 minute, the device will turn off
automatically
7 Slide the lock switch to the 'on' position to lock
the buttons.
If, after finishing the first measurement, another
measurement is required, do not lock the buttons
but press the user ID button to select the correct
user profile and follow steps 2-7.
Note: Wait at least 3 minutes between
measurements. This allows your blood circulation
to recover.
The device can store results of 60 blood pressure
measurements for both user 1 and 2.
Transmit and store personal health data
in the app
Note: Your personal measurement data is only
stored and displayed in the Philips HealthSuite
health app.
1 Activate the Philips HealthSuite health app and
Bluetooth on your mobile device directly after a
measurement.
-Keep the mobile device and the blood
pressure monitor at transmission distance
(no more than 16 feet / 5 meters from each
other, in the same room).
2 Once successfully connected, the measurement
results are transmitted to the health app and
the Bluetooth symbol lights up.
- If the data transmission is successful, the
measurement results are displayed in
the health app.
- If the data transmission fails, 'the Bluetooth
symbol together with 'Err' is shown. The
pending measurement data will be transmitted
to your mobile device the next time it connects
with your blood pressure monitor. You can also
try to resend the data:
-Activate the Health app on your mobile
device.
-Press the user ID button or 'on' button to
switch on the blood pressure monitor.
-The measurement results will be
automatically sent to your mobile if the
device has been added in the app.
-When the blood pressure monitor connects
via Bluetooth to the app of a user, the
device will automatically select that user and
measurements can only be done for that
user.
Cleaning and storage
Caution: This device is not washable. Never
immerse the device in water and do not rinse it
under running water.
Caution: Avoid sudden movements and hard
contacts with objects.
Caution: Never use compressed air, scouring pads,
abrasive cleaning agents or aggressive liquids
such as petrol or acetone to clean the device.
1 Switch off the device and unplug the USB plug
from the USB port.
2 Use a slightly damp or dry cloth to wipe the
surface of the monitor (Fig. 18) and the whole
unit.
3 Store the device in a cool, dry, and ventilated
environment, where it will not be crushed,
banged or subject to damage. For further
information please refer to the transport and
storage specifications detailed in this manual.
This device has no other user-serviceable parts.
For assistance call 1-844-531-6861.
Accessories
Philips accessories may be purchased at a store
near you, or on our website
www.philips.com/store.
Disposal
This device contains a rechargeable battery which
must be disposed of properly. Contact your local
town or city officials for battery disposal
information. You can also call 1-800-8-BATTERY
or visit www.rbrc.com for battery drop-off
locations.
For assistance, visit our website
www.philips.com/support or call 1-844-531-6861
toll free.
Recalibration and information
This device is calibrated at the time of
manufacture. If this blood pressure monitor is used
according to instructions, recalibration will not be
needed for 5 years (10000 use cycles).
Recalibration can be carried out by an appropriate
authority or authorized service center. This
calibration will be charged for by said authority.
If you need more information about the app,
please visit www.philips.com/healthprograms

Assistance
For assistance, visit our website:
www.philips.com/support or call toll free
1-844-531-6861
Full Two-Year Warranty
Philips Electronics North America Corporation
warrants each new Philips product, model DL8765,
against defects in materials or workmanship for a
period of two years from the date of purchase and
agrees to repair or replace any defective product
without charge.
IMPORTANT: This warranty does not cover
damage resulting from accident, misuse or abuse,
lack of reasonable care, the affixing of any
attachment not provided with the product or loss
of parts or subjecting the product to any but the
specified voltage.*
NO RESPONSIBILITY IS ASSUMED FOR ANY
SPECIAL, INCIDENTAL OR CONSEQUENTIAL
DAMAGES.
In order to obtain warranty service, simply go to
www.philips.com/support or call toll-free
1-844-531-6861. It is suggested that for your
protection you return shipments of product by
insured mail, insurance prepaid. Damage occurring
during shipment is not covered by this warranty.
NOTE: No other warranty, written or oral, is
authorized by Philips Electronics North America
Corporation. This warranty gives you specific legal
rights, and you may also have other rights which
vary from state to state. Some states do not allow
the exclusion or limitation of incidental or
consequential damages, so the above exclusion
and limitations may not apply to you.
* Read enclosed instructions carefully.
Manufactured for: Philips Consumer Lifestyle, A
division of Philips Electronics North America
Corporation, P.O. Box 10313, Stamford, CT 06904.
PHILIPS and Philips Shield are registered
trademarks of Koninklijke Philips N.V.
Troubleshooting
This chapter summarizes the most common
problems you could encounter with the device. If
you are unable to solve the problem with the
information below, visit www.philips.com/support
for a list of frequently asked questions or call
1-844-531-6861 for assistance.
Troubleshooting
Problem Possible
cause
Solution
My blood
pressure
fluctuates
throughout
the day.
Your
measurement
position, the
conditions
under which
you measure
or the time of
measureme
nt, are
different
during each
measureme
nt.
For a meaningful
comparison, try to
measure under
similar conditions.
For example, take
daily
measurements at
approximately the
same time, on the
same wrist, or as
directed by a
physician.
Fluctuations
of blood
pressure
during the
day are
normal.
Blood pressure
fluctuates from
minute to minute
and normally
shows a circadian
rhythm over a
24-hour period,
with highest
readings in the
afternoons and
lowest readings at
night. That is why,
for comparable
measurements, the
measurements
should be taken at
approx. the same
time of day.
You are using
medication.
The variations in
blood pressure can
be greater if you
are using
medication.
You
performed
multiple
measure
ments
directly after
each other.
Wait at least 3
minutes between
measurements.
This allows your
blood circulation to
recover.
My blood
pressure
measure
ment from
the hospital
is different
from the
measure
ment at
home.
Multiple
variables may
affect your
blood
pressure such
as the
weather,
emotions
and exercise.
Pay attention when
you measure your
blood pressure at
home. Check for
instance:
If the cuff is not too
tight or too loose.
If the cuff is
properly attached
on the wrist.
If you feel anxious
or stressed, try to
relax. Take a deep
breath 2-3 times
before you start a
measurement.
Advice: Rest for 5
minutes before you
measure your
blood pressure.
The result is
different
when I
perform
measure
ments on
my right
wrist.
The blood
pressure
monitor is
suitable to be
used on both
wrists, but the
measurement
results on the
right wrist
and left wrist
will differ.
For a meaningful
comparison, try to
measure under
similar conditions
and measure on
the same wrist
every time.
Problem Possible
cause
Solution
The blood
pressure
monitor
does not
work when I
press the
'on' button
The
rechargeable
battery is
empty.
Recharge the
battery (see
'Charging').
The lock
switch is set
to the
'locked'
postion.
Set the lock switch
to the 'unlocked'
position (Fig. 7).
The light of
the display
dims and a
battery
symbol+Lo
is showing
The battery is
low.
Charge the battery
(see 'Charging').
The display
shows Err
Communica
tion error.
Check if the app is
on and try data
transmission again.
The display
shows E3
The cuff is
not properly
secured.
Refasten the cuff,
wait for 3 minutes
and then measure
again.
The display
shows E10
or E11
The device
detected
motion,
talking or the
heart rate is
too weak
during the
measureme
nt.
Wait for 3
minutes and then
measure again. Do
not move during
measurement.
The display
shows E20
The device
does not
detect the
heart rate.
Make sure the
device is in contact
with the skin.
Loosen the
clothing on the arm
and measure again.
The display
shows E21
The
measurement
failed.
Wait for 3 minutes
and then measure
again.
The display
shows EExx
A system
error
occurred.
Retake the
measurement. If
the problem
persists, call
1-844-531-6861 for
assistance.
Data
transmission
or pairing
failed.
Bluetooth is
off.
Turn on Bluetooth
on your mobile
device.
The Philips
HealthSuite
health app is
off.
Press the icon on
your mobile device
to activate the
health app.
The blood
pressure
monitor and
mobile
device are
more than 16
feet/ 5
meters away
from each
other.
Place your mobile
device closer to the
blood pressure
monitor.
You selected
the wrong
profile on the
blood
pressure
monitor.
Select the correct
user profile on the
blood pressure
monitor before
your measurement.
Otherwise the data
cannot be
transmitted to your
app. Repeat the
measurement with
the correct profile
selected
Specifications
Product name Philips Wrist blood
pressure monitor with
Bluetooth® Smart
Power supply 3.7V 420mAH built-in
rechargeable li-polymer
battery
Display Display with white
backlight
Visible area = 1 13/16" (L)
x 1 3/16" (W) / 46 mm x
30 mm
Measurement method Oscillometric method
Measurement range Rated cuff pressure:
0mmHg - 300mmHg
Measurement pressure:
40mmHg - 230mmHg
heart rate: 40-199 beats
per minute
Accuracy Pressure: 41°F to 104°F
/5°C to 40°C within ±3
mmHg
heart rate: ±5% of
measurement result on
display
Normal operating
conditions
Temperature: 41°F to
104°F /5°C to 40°C
Relative humidity:
≤85%RH.
Atmospheric pressure:
86kPa to 106kPa
Storage and
transportation
conditions
Temperature: -4°F to
140°F /-20°C to 60°C
Relative humidity: 10% to
93%.
Atmospheric pressure:
50kPa to 106kPa
Measurement
perimeter of the wrist
About 5 5/16" - 8. 1/2" /
13.5 cm - 21.5 cm
Net weight Approx. 3.5 oz / 100g
External dimensions Approx. 3 1/8" x 2 1/2" x
1/2" / 79 mm x 64 mm x
13 mm
Accessories USB cable, user manual
Mode of operation Continuous operation
Degree of protection Type BF applied part
Protection against
ingress of water
IP22, This means:
protected against access
to hazardous parts with a
finger and against
vertically falling water
drops when tilted up to
15 degrees.
Device classification Battery Powered Mode:
Internally Powered ME
Equipment. Class II ME
Equipment
Caution: No modification of this equipment is
allowed.
Explanation of symbols
The warning signs and symbols are essential to
ensure that you use this product safely and
correctly and to protect you and others from injury.
Below you find the meaning of the warning signs
and symbols on the label and in the user manual.
Symbol for 'follow instructions for use'.
This symbol means that the part of the
device that comes into physical contact
with the user (also known as the applied
part) is of type BF (Body Floating)
according to IEC 60601-1. The applied
part is the cuff.
Compliant with the Waste Electrical and
Electronic Equipment/Restriction of the
Use of Certain Hazardous Substances in
Electrical and Electronic Equipment
(WEEE) recycling directives.
Indicates manufacturing date.
Symbol for 'direct current'.
Symbol for the 'Bluetooth Combination
mark'. The device uses Bluetooth for
communication.
Indicates the manufacturer's serial
number so that a specific medical
device can be identified.
Indicates manufacturer's catalog
number of the appliance.
Fuse T1A/250V Φ3.6*10CCC.
Symbol for 'Class II Equipment'.
Symbol for indoor use only.
Symbol for 'Including RF transmitter'.
This means that this device emits non-
ionizing radiation. All devices with RF
transmitters or that use RF
electromagnetic energy must have a
label with this symbol.
Indicates caution.The user should
consult the instructions for use for
important cautionary information such
as warnings and precautions that
cannot, for a variety of reasons, be
presented on the medical device itself.
Symbol for micro-USB connector.
Symbol for USB connector.
This symbol on the device means:
protected against access to hazardous
parts with a finger and against vertically
falling water drops when tilted up to 15
degrees.
–4ºF
140ºF
–4ºF
140ºF
Indicates the storage and transportation
temperature limits to which the medical
device can be safely exposed: -4°F to
140°F / -20°C to 60°C.
Indicates the relative humidity limits to
which the device can be safely exposed:
10% to 93%.
Symbol for the 2 year Philips warranty.
This appliance contains a rechargeable
battery which must be disposed of
properly. See chapter ' Disposal' for
more information.
Electromagnetic emissions and
immunity
The device is approved according to EMC safety
standard IEC 60601-1-2. It is designed to be used
in typical domestic environments.
EMC Guidance
- The Blood Pressure Monitor needs special
precautions regarding EMC and needs to be
installed and put into service according to the
EMC information provided in the accompanying
documents.
- Wireless communications equipment such as
wireless home network devices, mobile phones,
cordless telephones and their base stations,
walkie-talkies can affect this equipment and
should be kept at least a distance equivalent to
3.3m (11 ft) away from the equipment.
Note: As indicated in IEC 60601-1-2:2007 for ME
equipment, a typical cell phone with a maximum
output power of 2 W yields equivalent to 3.3m (11
ft) at an immunity level of 3V/m.
Table 1 Guidance and manufacturer's
declaration – electromagnetic emissions
- for all ME equipment and ME systems
Guidance and manufacturer’s declaration –
electromagnetic emissions
The device is intended for use in the
electromagnetic environment specified below. The
customer or the user of the device should assure
that it is used in such an environment.
Emissions test Com
pliance
Electromagnetic
environment -
guidance
RF emissions
CISPR 11
Group 1 The device must emit
electromagnetic
energy in order to
perform its intended
function. Nearby
electronic equipment
may be affected.
RF emissions
CISPR 11
Class B
Harmonic
emissions IEC
61000-3-2
Not
applica
ble
Voltage
fluctua
tions/flicker
emissions IEC
61000-3-3
Not
applica
ble
Table 2 Guidance and manufacturer's
declaration – electromagnetic immunity
– for all ME equipment and ME systems
Guidance and manufacturer’s declaration –
electromagnetic immunity
The device is intended for use in the
electromagnetic environment specified below. The
customer or the user of the device should assure
that it is used in such an environment.
Immuni
ty test
IEC
60601
test
level
Com
pliance
level
Electromagnetic
environment -
guidance
Electro
static
dis
charge
(ESD)
IEC
61000-
4-2
±6 kV
contact
±8 kV air
±6 kV
contact
±8 kV
air
Floors should be
wood, concrete or
ceramic tile. If
floors are covered
with synthetic
material, the
relative humidity
should be at least
30%.
Electri
cal fast
tran
sient/b
urst IEC
61000-
4-4
±2 kV for
power
supply
lines
±1 kV for
input/o
utput
lines
±2 kV
for
power
supply
lines
Electrical power
quality should be
that of a typical
commercial or
hospital
environment.
Surge
IEC
61000-
4-5
±1 kV
line(s) to
line(s)
±2 kV
line(s) to
earth
±1 kV
line(s)
to
line(s)
Electrical power
quality should be
that of a typical
commercial or
hospital
environment.
Voltage
dips,
short
interrup
tions
and
voltage
varia
tions on
power
supply
input
lines IEC
61000-
4-11
<5% UT
(>95%
dip in
UT) for
0.5
cycle
40% UT
(60%
dip in UT
) for 5
cycles
70% UT
(30% dip
in UT )
for 25
cycles
<5% UT
(>95%
dip in UT
) for 5 s
<5% UT
(>95%
dip in
UT) for
0.5
cycle
40% UT
(60%
dip in
UT ) for
5
cycles
70% UT
(30%
dip in
UT ) for
25
cycles
<5% UT
(>95%
dip in
UT ) for
5 s
Electrical power
quality should be
that of a typical
commercial or
hospital
environment. If the
user of the device
requires continued
operation during
power
interruptions, it is
recommended
that the device be
powered from an
uninterruptible
power supply or a
battery.
Power
fre
quency
(50/60
Hz)
magnet
ic field
IEC
61000-
4-8
3A/m 3A/m Power frequency
magnetic fields
should be at levels
characteristic of a
typical location in
a typical
commercial or
hospital
environment.
Note: UT is the AC electrical voltage prior to
application of the test level.
Table 4 Guidance and manufacturer's
declaration – electromagnetic immunity
–for ME equipment and ME systems that
are not life supporting
Guidance and manufacturer’s declaration –
electromagnetic immunity .The device is intended

for use in the electromagnetic environment
specified below. The customer or the user of the
device should assure that it is used in such an
environment.
IMMUNITY
test
IEC 60601 TEST
LEVEL
Compliance
level
Conducted RF
IEC
61000-4-6
3 Vrms
150 kHz to
80 MHz
3 Vrms
Radiated RF
IEC
61000-4-3
3 V/m
80 MHz to 2.5
GHz
3 V/m
Electromagnetic environment - guidance
Portable and mobile RF communications
equipment should be used no closer to any part of
the device, including cables, than the
recommended separation distance calculated from
the equation applicable to the frequency of the
transmitter.
Recommended separation distance:
d = 1.167 ÖP
d = 1.167 ÖP 80 MHz to 800MHz
d = 2.333 ÖP 800 MHz to 2.5 GHz
where P is the maximum output power rating of
the transmitter in watts (W) according to the
transmitter manufacturer and d is the
recommended separation distance in metres (m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey (a),
should be less than the compliance level in each
frequency range (b).
Interference may occur in the vicinity of equipment
marked with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher
frequency range applies.
NOTE 2 These guidelines may not apply in all
situations. Electromagnetic propagation is affected
by absorption and reflection from structures,
objects and people.
(a) Field strengths from fixed transmitters, such as
base stations for radio (cellular/cordless)
telephones and land mobile radios, amateur radio,
AM and FM radio broadcast and TV broadcast
cannot be predicted theoretically with accuracy. To
assess the electromagnetic environment due to
fixed RF transmitters, an electromagnetic site
survey should be considered. If the measured field
strength in the location in which the device is used
exceeds the applicable RF compliance level
above, the device should be observed to verify
normal operation. If abnormal performance is
observed, additional measures may be necessary,
such as re-orienting or relocating the device.
(b) Over the frequency range 150 kHz to 80 MHz,
field strengths should be less than 3V/m.
Table 6 Recommended separation
distances between portable and mobile
RF communications equipment and the
ME equipment or ME system – for ME
equipment and ME systems that are not
life supporting
Recommended separation distances between
portable and mobile RF communications
equipment and the device.
The device is intended for use in an
electromagnetic environment in which radiated RF
disturbances are controlled. The customer or the
user of the device can help prevent
electromagnetic interference by maintaining a
minimum distance between portable and mobile
RF communications equipment (transmitters) and
the device as recommended below, according to
the maximum output power of the
communications equipment.
Separation distance according to
frequency of transmitter (m)
Rated
maximum
output
power of
transmitter
(W)
150 kHz to
80 MHz
d = 1.167 Ö
P
80 MHz
to 800
MHz
d = 1.167 Ö
P
800 MHz
to 2.5 GHz
d = 2.333
0.01 0.117 0.117 0.233
0.1 0.369 0.369 0.738
1 1.167 1.167 2.333
10 3.690 3.690 7.378
100 11.67 11.67 23.33
For transmitters rated at a maximum output power
not listed above, the recommended separation
distance d in metres (m) can be estimated using
the equation applicable to the frequency of the
transmitter, where P is the maximum output power
rating of the transmitter in watts (W) according to
the transmitter manufacturer.
NOTE 1 At 80MHz and 800MHz, the separation
distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all
situations. Electromagnetic propagation is affected
by absorption and reflection from structures,
objects and people.
FCC Compliance information
This device complies with Part 15 of the FCC Rules.
Operation is subject to the following two
conditions: (1) this device may not cause harmful
interference, and (2) this device must accept any
interference received, including interference that
may cause undesired operation.
FCC ID 2AEFK-DL8765
Radio interference
This equipment has been tested and found to
comply with the limits for a Class B digital device,
pursuant to Part 15 of the FCC Rules. These limits

are designed to provide reasonable protection
against harmful interference in a residential
installation. This equipment generates, uses and
can radiate radio frequency energy and, if not
installed and used in accordance with the
instructions, may cause harmful interference to
radio communications. However, there is no
guarantee that interference will not occur in a
particular installation.
If this equipment does cause harmful interference
to radio or television reception, which can be
determined by turning the equipment off and on,
the user is encouraged to try to correct the
interference by one or more of the following
measures:
- Reorient or relocate the receiving antenna.
- Increase the separation between the
equipment and receiver.
- Connect the equipment to an outlet on a circuit
different from that to which the receiver is
connected.
- Consult the dealer or an experienced radio/TV
technician for help.
RF Radiation exposure statement
This equipment complies with FCC RF radiation
exposure limits set forth for an uncontrolled
environment. For handheld/body-worn operation,
this equipment has been tested and meets the
FCC RF exposure guidelines. This transmitter must
not be co-located or operating in conjunction with
any other antenna or transmitter. Use of other
accessories may not ensure compliance with FCC
RF guidelines.
Do not attempt to repair or modify this equipment.
Any repairs or alterations made by the user to the
equipment may void the warranty and compliance
of the equipment. Changes or modifications made
to this equipment not expressly approved by
Philips may void the FCC authorization to operate
this equipment. For assistance visit our website
www.philips.com/support or call toll-free
1-844-531-6861.
BlueTooth wordmark
The BlueTooth® Smart wordmark and logos are
registered trademarks owned by Bluetooth SIG,
Inc. and any use of such marks by Philips is under
license.
App Store and iPhone
App Store and iPhone are trademarks of Apple
Inc., registered in the U.S. and other countries. App
Store is a service mark of Apple Inc.
Google Play and Android
Google Play and Android are trademarks of Google
Inc.