Spinal Modulation orporated MN0700 Clinical Programmer User Manual LP0015 Rev Draft FCC PProg
Spinal Modulation, Incorporated Clinical Programmer LP0015 Rev Draft FCC PProg
Contents
- 1. Revised user manual MN-0600
- 2. Revised user manual MN-0700
Revised user manual MN-0600

PatientProgrammer
ModelMN0600
UserManual
SpinalModulation,Inc.
1135O’BrienDrive
MenloPark,CA94025USA
CAUTION–InvestigationalDevice
LimitedbyFederal(US)LawtoInvestigationalUse
Copyright©June2011bySpinalModulation,Inc.AllRightsReserved.Noportionofthismanualmaybereproducedortransmitted
inanyformorbyanymeans,electronicormechanical,includingphotocopying,recording,oranyinformationstorageandretrieval
systems,withouttheexpresswrittenpermissionofSpinalModulation,Inc.
LP0015 Rev E, June 2011

SPINAL MODULATION, INC.
PATIENT PROGRAMMER USER MANUAL
2
TABLEOFCONTENTS
EXPLANATIONOFSYMBOLSONPRODUCTORPACKAGELABELING....................................................................... 3
GLOSSARY .............................................................................................................................................. 4
INTRODUCTION........................................................................................................................................ 5
INDICATIONSFORUSE ............................................................................................................................... 5
DESCRIPTION........................................................................................................................................... 5
WARNING .............................................................................................................................................. 5
PRECAUTIONS–FORYOURPATIENTPROGRAMMERANDYOURSTIMULATORDEVICE............................................. 6
PRECAUTIONS–FORYOURTHERAPY............................................................................................................ 7
PATIENTPROGRAMMERFEATURES ............................................................................................................ 10
CHARGINGTHEBATTERY.......................................................................................................................... 10
PROGRAMMERPOWERUP....................................................................................................................... 10
MAINMENU......................................................................................................................................... 12
CONNECTINGTOYOURSTIMULATORDEVICE................................................................................................ 13
PAINCONTROLSCREEN ........................................................................................................................... 15
BACKTOMAINMENU ........................................................................................................................ 15
SELECTGROUP .................................................................................................................................. 15
PROGRAMMERSTATUSBAR ................................................................................................................. 16
ADJUSTINGYOURSTIMULATORDEVICESETTINGS ......................................................................................... 16
TURNINGOFFSTIMULATION ................................................................................................................. 16
PAINCONTROLTAB............................................................................................................................ 17
ADJUSTSTIMULATIONLEVELFORABODYREGION .......................................................................................17
INFOTAB ......................................................................................................................................... 18
DEVICETABANDPHYSICIANTAB ...............................................................................................................18
CLINICTAB ............................................................................................................................................ 18
USINGYOURTNSDEVICE.................................................................................................................... 19
USINGYOURINSDEVICE......................................................................................................................... 19
AUTHORIZEDEUROPEANREPRESENTATIVE .................................................................................................. 19
COMPANYCONTACTINFORMATION ........................................................................................................... 19
GUIDANCEANDMANUFACTURER’SDECLARATION.......................................................................................... 20
ELECTROMAGNETICEMISSIONS............................................................................................................... 20
ELECTROMAGNETICIMMUNITY .............................................................................................................. 21
APPENDIXI:INSBATTERYLIFE ................................................................................................................. 23
APPENDIXII:TROUBLESHOOTING .............................................................................................................. 32
3
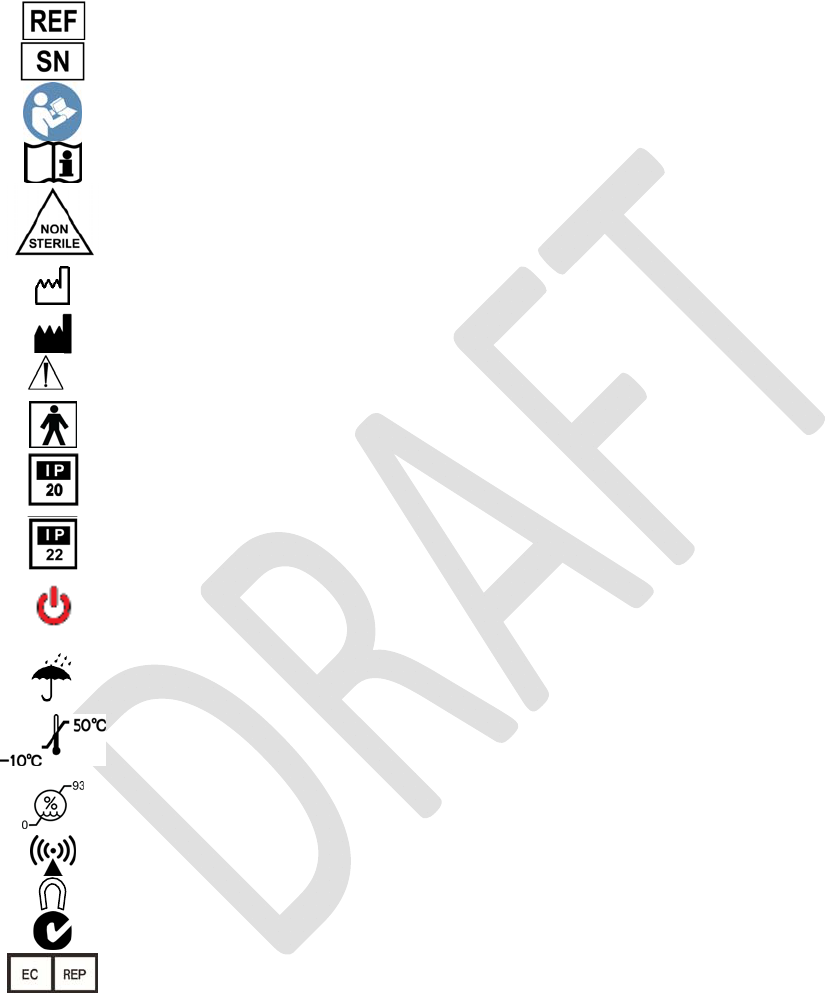
Explanation of Symbols on Product or Package Labeling
ModelNumber
SerialNumber
ReadtheManual
ConsulttheManual
ContentsofPackageareNon‐Sterile
ManufacturingDate
Manufacturer
Warning.Payattention.
ProtectedagainstElectricShock
Notwaterproof.AppliestotheProgrammerwhenitisnotinits
carryingcase.
Limitedwaterproof.AppliestotheTNS.
AppliestotheProgrammerinitscarryingcase.
TurnstheProgrammerONandOFF.
TurnsstimulationOFFontheTNS.
KeepDry
Storebetween‐10°Cand50°C(14°Fand122°F)
Storebetween0and93%humidity
Thedeviceisaradiotransmitter
Magnet.ShowsthelocationoftheProgrammermagnet.
AustraliaC‐tick
AuthorizedEuropeanRepresentative

4
Glossary
Lead–Surgicalwire:takeselectricalsignalsfromtheneurostimulatortothestimulationarea.
Stimulation–Smallelectricalpulses:producesatinglingsensationandreplacespainsignals.
Stimulator–Devicethatmakeselectricalpulsesthatstimulatethenervesinyourspine:definedasTrial
NeurostimulatororImplantableNeurostimulator.
TrialNeurostimulator(TNS)‐Externalstimulatordevicethatclipsontoyourbelt:attachestotheconnector
cableandwhichisconnectedtoleadsimplantedintheareanearyourspine.
ImplantableNeurostimulator(INS)‐Stimulatordeviceimplantedinyourbackorabdomen:attachestoleads
implantedintheareanearyourspine.
ConnectorCable–Cablethatconnectstheleadstoyourtrialstimulatordevice.
PatientProgrammer–Portable,hand‐helddevice:allowsyoutoadjustthestimulationsettings.
ClinicalProgrammer–Portable,hand‐helddevice:allowsthecliniciantoprogramthestimulatordevice.
ComputerTomography(CT)Imaging–ComputerizedX‐rayimaging:produceselectronicimagesoftissues
andorgans.
Diathermy–Highenergyheat:usedtocutorcauterizeduringsurgeryoratypeoftherapy.
ElectromagneticInterference(EMI)–Electricalsignalsthatinterferewiththedevicefunction.
MagneticResonanceImaging(MRI)–Medicalimaging:produceselectronicimagesoftissuesandorgans.
Paresthesia–Tinglingsensationfeltduringtherapydelivery:producedbyspinalcordstimulation.
Precaution–Situationthatcouldcauseuncomfortablestimulationandpossibledamagetothestimulator
deviceorPatientProgrammer.
Program–InstructionsorchangestostimulationsettingsthatareprogrammedintothePatientProgrammer
andtransmittedtothestimulatordevice.
StimulationLevel–Measureofstimulation:canbeincreasedordecreasedwithinarangespecifiedbyyour
doctor.
Warning–Potentiallyserioushazardthatcouldcauseinjuryordeath.

5
INTRODUCTION
YourPatientProgrammerisusedtoprogramyourstimulatordevice,ifrequired.ThisUserManualgives
detailedinstructionsonhowtosafelyuseyourPatientProgrammerandyourstimulatordevice.Italso
instructsyouonhowtorechargeyourPatientProgrammer.Seeyourdoctorifyouhaveanyquestions.
INDICATIONSFORUSE
TheSpinalModulationNeurostimulatorSystemwasdesignedtobeusedtomanageprolongedpainas
statedinthefollowingindicationstatement:
TheSpinalModulationneurostimulationsystemisindicatedasanaidinthemanagementofchronic,
intractable,neuropathicpainofthelowerlimbs‐includingunilateralorbilateralpain,associatedwiththe
followingconditions:radicularpain,peripheralneuropathies.
DESCRIPTION
TheClinicalandPatientProgrammersareusedtoconnecttothestimulatordevice.
– TheClinicalProgrammercontrolsthestimulatordevice.Onlyyourdoctorand/orSpinalModulation
clinicalpersonnelmayusetheClinicalProgrammer.
– ThePatientProgrammerallowsyouto
adjustthesettingsofthestimulator
device.Yourdoctorspecifiestherangeof
settings.ItalsoallowsyoutoturnOFFall
stimulation,ifrequired.
ThePatientProgrammerisaportable,handheld
device.Itisdesignedtobeeasytouse.Itcanbe
pluggedintoapoweroutletorpoweredbyan
internal,rechargeablebattery.ThePatient
Programmerusesaninternalmagnettoconnect
tothestimulatordevice.Thisallowsthepatient
tocontrolstimulationsettings.
WARNING
• DonotuseyourPatientProgrammerorthestimulatordeviceuntilyourdoctorhastrainedyou.
• DonotuseyourPatientProgrammeruntilyourdoctorhassetupyourstimulatordevice.
• Donotundergoanyelectivemagneticresonanceimaging(MRI)procedure.IfMRIisnecessary,your
physicianmustremoveanylead(s).YourdoctormustalsodisconnecttheTNSorINSdevice.Useof
MRIintheareaofthelead(s)maydislodgethelead(s)ordamagetheTNSorINS.Ifavoltageis
inducedthroughthelead,itmaycauseuncomfortable(“jolting”or“shocking”)levelsofstimulation.
• Donotundergoanydiathermy(highenergyheat)procedures.Diathermymaycausebodilyinjuryor
damagetothestimulatordevice.
• Donotremovethelead(s)orConnectorCableduringthetrialperiod.Aninfectionmayresult.
• Changesinbodypositioncanincreasepainorcauseuncomfortablestimulation.UsethePatient
ProgrammertoadjuststimulationlevelsortoturnOFFstimulation,ifrequired.

6
Warnings(continued)
• Strongelectromagneticfieldsmayinterferewiththestimulatordevice.Thisinterferencecanaffect
thestimulationlevelandcausediscomfort.Avoidtheftdetectiondevicesatstoreandlibraryexits.
Alsoavoidairportsecurityscreeners.Donotstandnearthescreeningequipment.
• Otherequipmentthatmaycauseinterferenceincludesbutisnotlimitedto:powergenerators,arc
weldersandlargemagnetizedspeakers.Donotstandnearthesedevices.
• DonotleaveyourProgrammerChargerwherepets,childrenoryoumaybecomeentangledinthe
cord,causingafallorstrangulation.
• Reportarashduetosystemcomponentstoyourdoctor.Ifyourthroatortonguestartstoswellget
emergencyaidimmediately.
PRECAUTIONS–FORYOURPATIENTPROGRAMMERANDYOURSTIMULATORDEVICE
FollowtheseprecautionstoavoiddamagetoandassureproperfunctionofyourPatientProgrammerand
stimulatordevice.
• DonotdropormishandleyourPatientProgrammerorstimulatordevice.Physicaldamagetothe
devicesmayimpairtheirfunction.
• DonotwashthePatientProgrammerorTNSdevicewithexcessivewater.Donotgeteitherdevice
wet.Excessivemoisturemayimpairtheirfunction.Ifcleaningisnecessary,removesoilwithasoft
dampcloth.
• DonotshowerorbathewiththeTNSdevice.(YoumaytakeaspongebathiftheTNSdevicedoes
notgetwet.)
• AvoidcontactwithbodyfluidsfortheTNSandPatientProgrammer.Contaminationmaycause
damagetothedevices.
• DonotuseabrasiveorcausticcleaningproductsonyourPatientProgrammerorTNSdevice.
• DonotuseanyequipmentoraccessoriesthatarenotsuppliedwithyourPatientProgrammer.Do
notpluganythingintotheconnectoratthebottomoftheprogrammer.ItisforClinicuseonly.
• DonotopenthecasesofthePatientProgrammerorTNSdeviceormodifytheminanyway.This
mayexposethedevicestoelementsthataltertheirfunction.
• DonotplaceyourPatientProgrammerclosetocreditcardsorothercardswithmagneticstrips.
ThePatientProgrammermagnetmaydemagnetizeyourcards.KeepthePatientProgrammer
awayfromcomputerharddrivesandmagneticstoragedevices.
• DonotoperatethePatientProgrammerorstimulatordeviceoutsidethetemperaturerangeof‐
5°Cto45°C(23°Fto113°F).Rapidtemperaturechangesmayaffectproperdeviceoperation.
• DonotstorethePatientProgrammeroutsidethetemperaturerangeof‐10°Cto50°C(14°Fto
122°F).
• DonotleavethePatientProgrammerinacarorotherplaceswheretemperaturescanexceed
50°C(122°F).

7
DevicePrecautions(continued)
• Failureofyourstimulatorsystem,althoughunlikely,ispossibleduetorandomcomponentfailure.
Ifanypartofyourstimulatorsystemstopsworkingoryouseeachangeinhowitworks,
discontinueuseandcontactyourdoctorduringnormalbusinesshours.
• ReturnyourPatientProgrammerandTNSdevicetoyourdoctorattheendofthetrialperiod.Do
notdiscardorburnthePatientProgrammerorTNSdevice.Firemaycausetheinternalbatteries
toexplode.
• DonottrytoreplacetheTNSdevicebattery,eveniftheTNSdevicedoesnotfunction.OnlySpinal
ModulationpersonnelmayreplacetheTNSdevicebattery.
• Donotuseanyothercompany’sdevicetoprogramyourstimulatordevice.UseonlythePatient
ProgrammerprovidedbySpinalModulation.
• DonotallowunauthorizeduseofyourPatientProgrammer.Thismaycauseunwanted
programmingchanges.
• DonotusethePatientProgrammerorstimulatordevicenearexplosiveorflammablegases.
Seriousinjurymayoccur.
• DonotusetheProgrammerChargerifthepowercordisdamaged,excessivelywornorfrayed.
Thismaycauseinjuryordamageyourstimulationdevice.
• ToremovepowerfromtheProgrammerChargerwhennotinuse,unplugfromthewall.
• Frequentprogrammingofyourimplanteddevicewillcausethebatterytodepletefaster.Avoid
unnecessaryprogramming.
PRECAUTIONS–FORYOURTHERAPY
Followtheseprecautionstomaintainappropriatetherapy:
• Followproperwoundcaretechniques,asinstructedbyyourdoctor.
• Donotruborpressontheimplantsite.Thismaycausetheleadstodislodgeoryourskintoerode.
ItmayalsocauseinversionoftheINS.
• Avoidexcessivebending,twistingandstretching.Donotliftobjectsovertenpounds.These
activitiesmaycausetheleadstomove.Understimulationoroverstimulationmayresult.
• AvoiddrivingacaroroperatingotherpotentiallydangerousmachinerywhilestimulationisON.
Youcouldbedistractedfromvehicleordeviceoperationifsuddenchangesinstimulationwereto
occur.
• Yourstimulatordevicemayaffecttheoperationofotherimplantabledevices,suchaspacemakers
orimplantablecardiacdefibrillators.Tellyourdoctoraboutanyotherimplantabledevicesthat
youhaveorarescheduledtoget.

8
TherapyPrecautions(continued)
• Tellyourregulardoctor(s)orhealthcareprovidersthatyouhaveastimulatordevice.Donot
undergoanyelectivemedicalprocedureswithoutfirstdiscussingthemwithyourphysician.Some
medicaldevicesortherapies,suchasthoselistedbelow,mayinterferewithyourstimulator
device:
o Electrocautery–Electricprobetocauterizebloodvesselsandstopbleedingduringsurgery.
o Lithotripsy–Shockwavestobreakupgallstonesandkidneystones.
o TherapeuticRadiation–Usedtodestroycancercells.
o High‐outputultrasound–Soundwavestotreatboneandmuscleinjuries,ortostimulate
muscleorimprovebloodflow.
o RFAblation–Radiofrequencyenergytocausecontrolledtissuedamage.
o MicrowaveAblation–Alternatingelectricfieldtocausecontrolledtissuedamage.
o Dentalprocedures,electrolysis,staticfieldtherapeuticmagnetsanddiagnosticX‐ray.
• Appointafamilymemberorfriendtotellemergencymedicalpersonnelthatyouhavea
stimulatordevice,incaseyouneedemergencycare.YouwillbegivenaMedicalAlertCardto
carrywithyou.Thiscardwillinformemergencymedicalpersonnelthatyouhaveastimulator
device.
Ifyouhaveanyconcernsaboutyourstimulatordevice,contactyourdoctorduringnormalbusinesshours.
RFOperatingFrequencies
NearbyequipmentemittingstrongmagneticfieldscaninterferewithRFcommunication,eveniftheother
equipmentcomplieswithCISPRemissionrequirements.Theoperatingcharacteristicsareasfollows:
MICSband:402‐405MHz
Theeffectiveradiatedpowerisbelowthelimitsasspecifiedin
Europe:ENETSI301839‐2
USAFCC47CFRPart95;95.601‐95.673SubpartE,95.1201‐95.1219
FCCID:Y8L‐MN0700
Thisdevicemaynotinterferewithstationsoperatinginthe400.150–406.000MHzbandinthe
MeteorologicalAids,MeteorologicalSatellite,andEarthExplorationSatelliteServicesandmustacceptany
interferencereceived,includinginterferencethatmaycauseundesiredoperation.
.
9
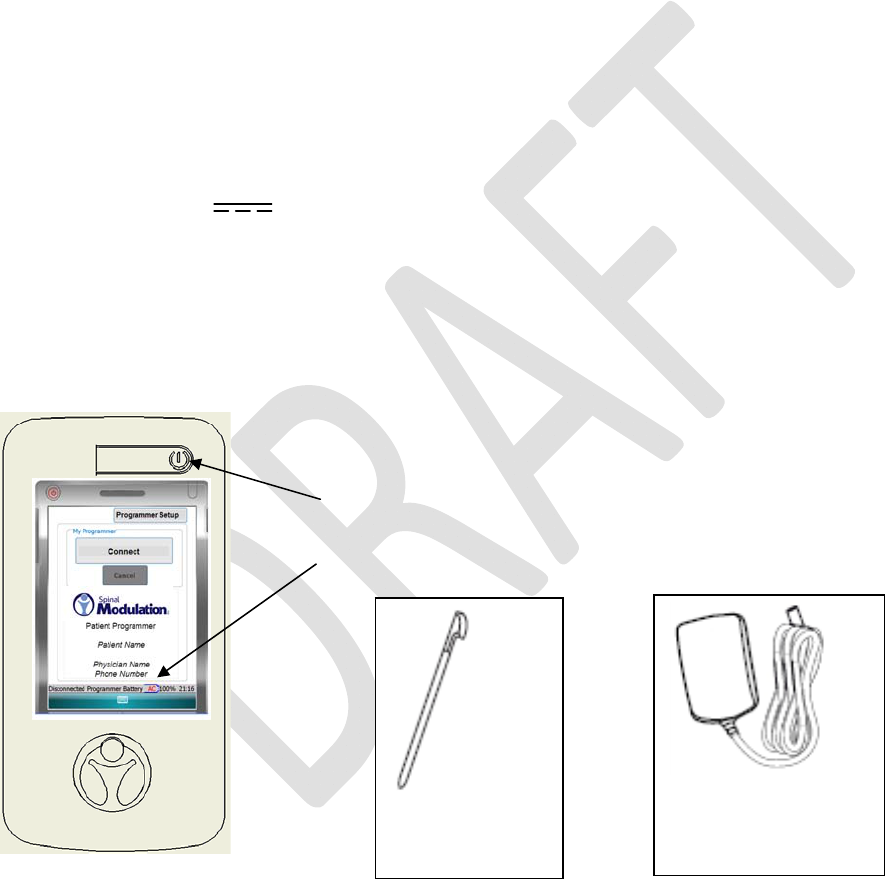
PATIENTPROGRAMMEROVERVIEW
YourPatientProgrammerisaportable,handhelddevice.Itispoweredbyaninternal,rechargeablebattery.
Itcanalsobepluggedintoapoweroutlet.YourPatientProgrammerworkswithyourstimulatordeviceto
controlstimulation.YourdoctorwillexplainhowtousethePatientProgrammertoadjuststimulationfor
optimalpainrelief.
KeepyourPatientProgrammernearyouatalltimes.Thisallowsyoutoadjuststimulation,ifnecessary.
CarryyourPatientProgrammerinthecaseprovidedasthecaseprovidesprotectionfromwater.
YourPatientProgrammersystemconsistsof:
• PatientProgrammerwithStylus(andinternalmagnet)MN0600
• AuxiliaryMagnetMN3300
• ProgrammerChargerMN3400
Input:100‐240VAC,50‐60Hz,0.6A
Output:5V 3.0A
• CarryingCaseMN3500
• PatientProgrammerUserManual(thisdocument)
• PatientInformationSheet
• PatientMedicalAlertCard
PatientProgrammer
Programmer
Charger
Programmer Power
Stylus
•Battery Status Bar
•AC – Battery Charging
•100% – Battery Charge Complete
10
PATIENTPROGRAMMERFEATURES
WithyourPatientProgrammer,youcan:
• TurnOFFallstimulation,ifrequired.
• TurnstimulationONorOFFforeachbodyregion.
• Adjustthestimulationlevelforeachbodyregion.
• ChangetheGroupforstimulation.See“SelectGroup”underthe“PainControlScreen”section.
• ViewyourstimulatordeviceIDinformation.
• ViewyournameoryourIDnumber.
• Viewyourleadimplantdate.
• Viewyourphysicianname,clinicnameandcontactinformation.
CHARGINGTHEBATTERY
YouwillneedtheProgrammerChargerprovidedtochargethePatientProgrammerbattery.Chargingthe
batterytakesapproximately2–4hoursforafullcharge.TheProgrammerStatusBaratthebottomofthe
screenshowsthebatterychargelevel.
1. ConnecttheProgrammerChargertoapoweroutlet.
2. ConnecttheChargertoyourPatientProgrammer.
Whenthebatteryischarging,thebatteryicononthescreenshows“AC”.Whenchargingiscomplete,100%
showsnexttothebatteryicon.
YourPatientProgrammerwilloperatenormallyanddoesnotusebatterypowerwhenitisconnectedtoa
poweroutlet.ConnectyourPatientProgrammertotheChargerandattachtoanoutletregularlytokeepit
charged.
YourPatientProgrammerandProgrammerChargerhaveanexpectedserviceperiodofuptothreeyears.
Improperchargingmayreducethisperiod.
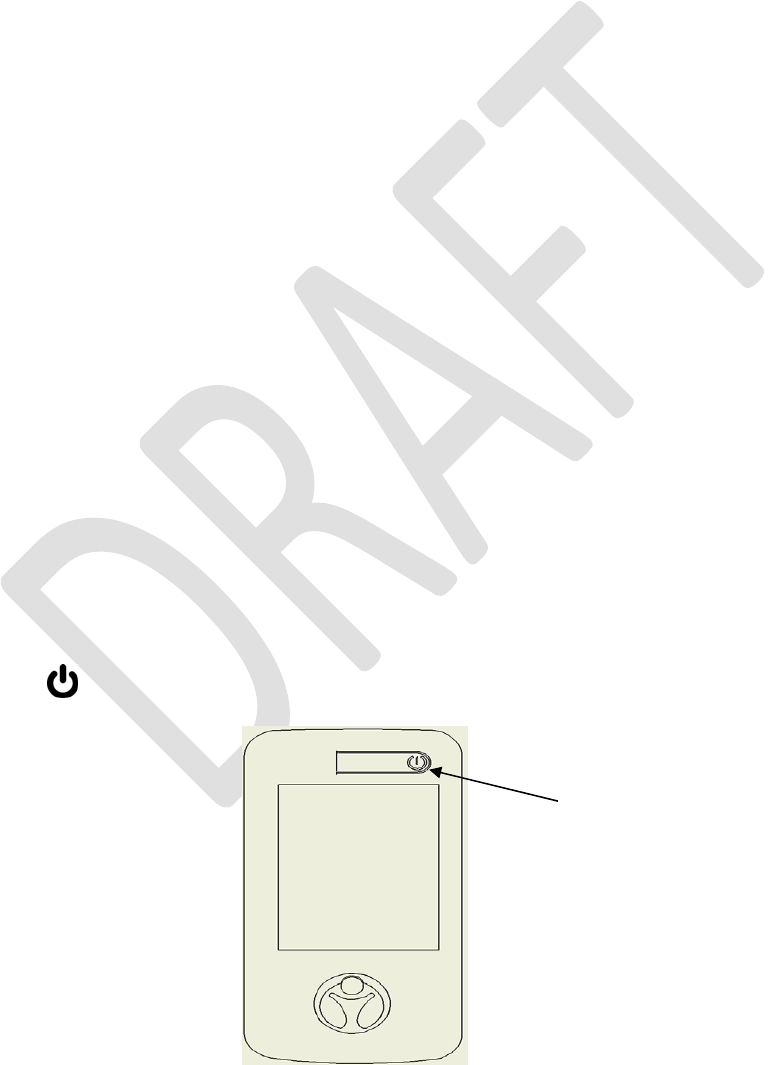
PROGRAMMERPOWERUP
Pressthe“”buttontoturnONyourPatientProgrammerscreen.TheMainMenuwilldisplay.
Press here to turn on
Programmer Screen

11
NOTE:IfthePatientProgrammerdoesnotturnON,chargethebattery,andtryagain.
12
MAINMENU
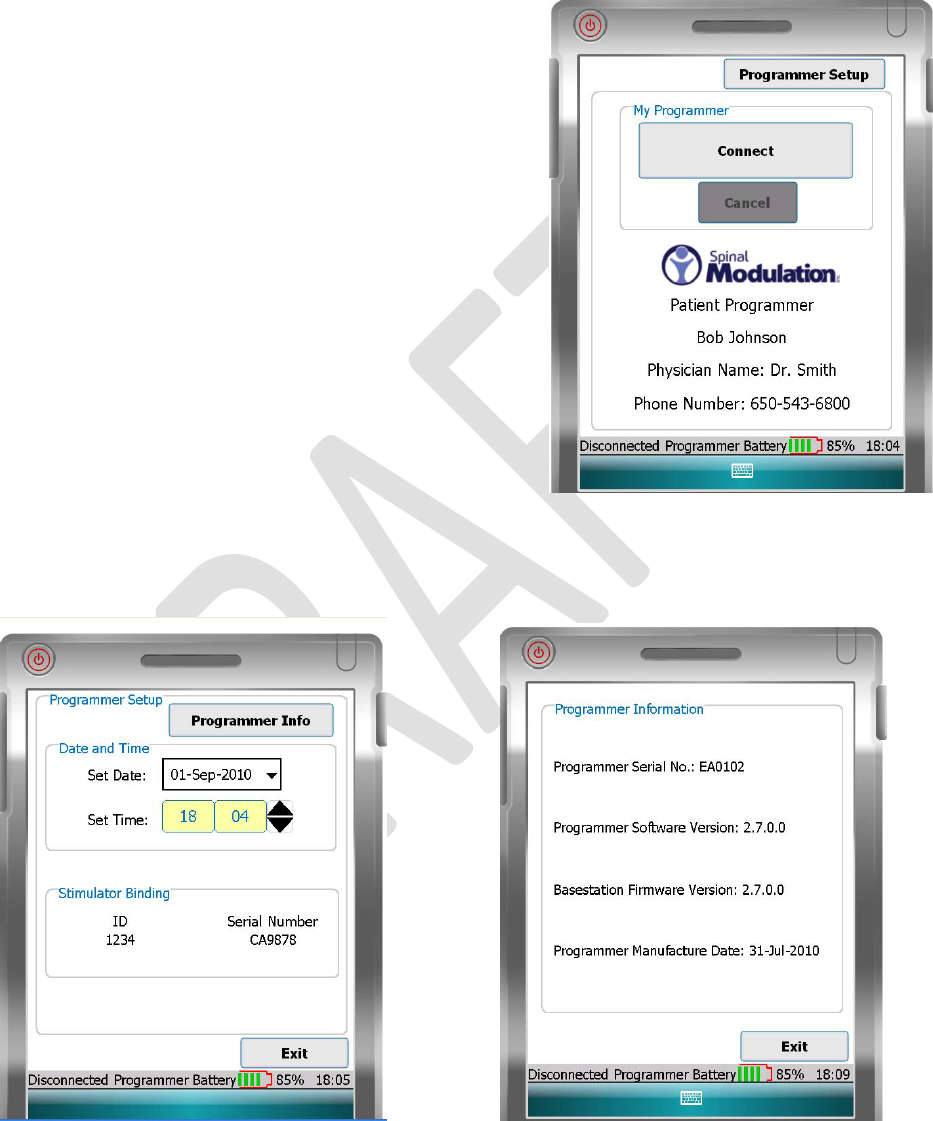
TheProgrammerMainMenudisplaystwomainfunctions:
• Connect:Allowsyoutoconnecttoyourstimulatordevice;
alsoallowsyoutoadjuststimulationsettings.
• ProgrammerSetup:AllowsyoutosetyourPatient
Programmerdateandtime;alsoallowsyoutoview
informationaboutyourstimulatordevice.
TheMainMenuidentifiesthedeviceasyourSpinalModulation
PatientProgrammer.Yourphysician,clinicandtheclinicphone
numberarealsoshown.
TheProgrammerstatusbarislocatedatthebottomoftheMain
Menu.ThestatusbarshowstheProgrammer–stimulator
connectionstatus.Italsoshowsthebatterychargelevelandthe
time.Seethe“ProgrammerStatusBar”sectioninthisUser
Manualformoredetail.
YoucanchangethetimeanddateandaccesstheProgrammer
InfoscreenfromtheProgrammerSetupscreen.Youcanalso
viewyourstimulatordeviceserialnumberandyournameor
patientID.
TheProgrammerInfoscreenshowsyourprogrammerserialnumber,softwareversionandmanufacturing
date.Italsoshowsthebasestationfirmwareversion.
13
StimulatorDeviceBinding
YourdoctorwillbindyourstimulatordevicetoyourPatientProgrammer.Youcannoteditthisinformation.
Connect
Pressthe“Connect”buttonontheMainMenutoconnecttoyourstimulatordevice.Onceconnected,you
canadjuststimulationsettings.Seethe“ConnectingtoYourStimulatorDevice”sectionbelowformore
detail.
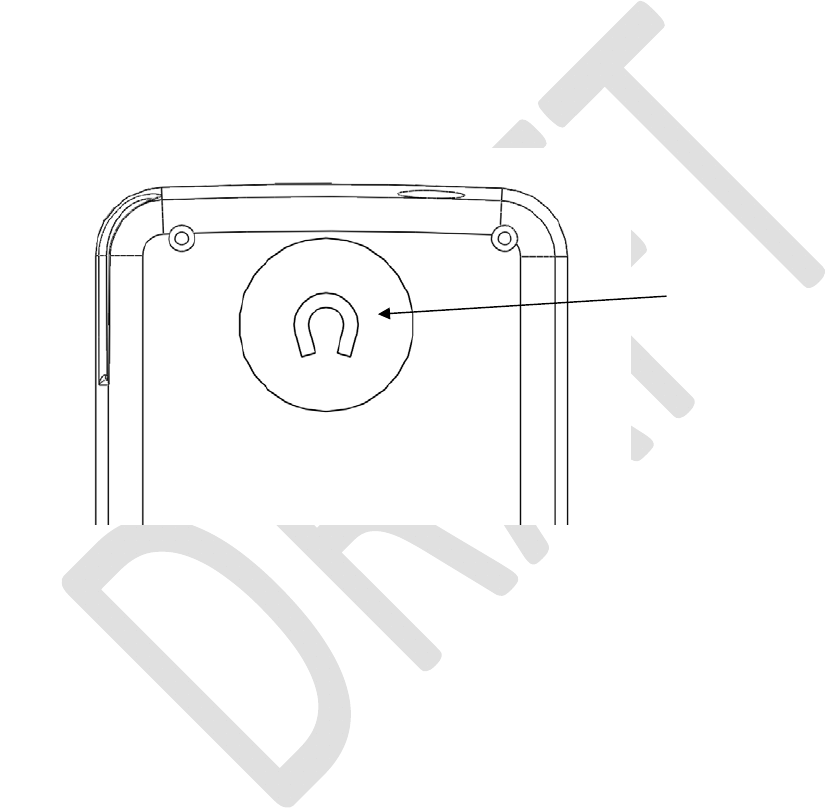
Magnet
AmagnetislocatedunderthemagnetsymbolonthebacksideofthePatientProgrammer.Placethe
magnetoverthestimulatordevicetocheckforconnectionbetweenthePatientProgrammerandstimulator
device.Seethe“ConnectingtoYourStimulatorDevice”sectionbelowformoredetail.
ConnectingtoYourStimulatorDevice
UseyourPatientProgrammertoconnecttoyourstimulatordevice.
• MakesurethatyourPatientProgrammeristurnedONandthattheMainMenudisplays.YourPatient
Programmermustbewithin6feetofyourstimulatordevice.
• Pressthe“Connect”button.ThePatientProgrammerwillbeginsearchingforthestimulatordevice.An
iconshowsonthescreentoindicatethatitisbusy.
• PositionthemagnetonthePatientProgrammeroveryourstimulatordeviceandmovearoundina
circularmotiontoinitiatecommunication.
ThePatientProgrammerchimeswhenitisconnectedtoyourstimulatordevice.ThePainControlscreen
displays.“Connected”showsinthestatusbaratthebottomleftofthescreen.IfyourPatientProgrammer
cannotconnecttoyourstimulatordevice,anerrormessagedisplays.“Disconnected”showsinthestatus
bar.
IfyourPatientProgrammercannotconnecttoyourstimulatordevice,gobacktotheMainMenu.Press
“Connect”again.Movethemagnetinacircularfashionoveryourstimulatordevice.RepeatuntilthePatient
Programmerconnectstothestimulatordevice.
NOTE:Ifafter2minutesyourPatientProgrammercannotconnecttothestimulatordevice,placethe
PatientProgrammeroverthestimulatordeviceagain.Anerrormessagemaydisplay.Themessageasksyou
Magnet

14
toconfirmthatyourPatientProgrammerisnearenoughtothestimulatordevice.Afterconfirmingthatyour
PatientProgrammeriswithin6feetofthestimulatordevice,press“OK”.Press“Connect”again.
15
PAINCONTROLSCREEN
AtthetopofthePainControlscreen.TheIDHeadingshows
yournameorIDnumberandyourstimulatordevice’sserial
number.
The“TurnAllStimulationOFF”buttonisbelowtheID
Heading.
Twotabsarebelowthe“TurnAllStimulationOFF”button:the
“PainControl”tabandthe“Info”(Information)tab.Seethe
“AdjustingYourStimulatorDeviceSettings”sectioninthis
UserManualformoredetail.
The“Exit”buttonatthebottomrightsideofthescreen
returnsyoutotheMainMenu.
IDHeading,locatedatthetopofthescreen,showsthe
followinginformation:
• YourIdentification(ID)Number
• YourStimulatorDeviceSerialNumber
BACKTOMAINMENU
The“Exit”buttonclosesthePainControlwindow,endsthe
session,andreturnstotheMainMenu.
NOTE:Whenprogrammingiscomplete,selectthe“Exit”
buttonandpowerofftheprogrammertoconservepower.
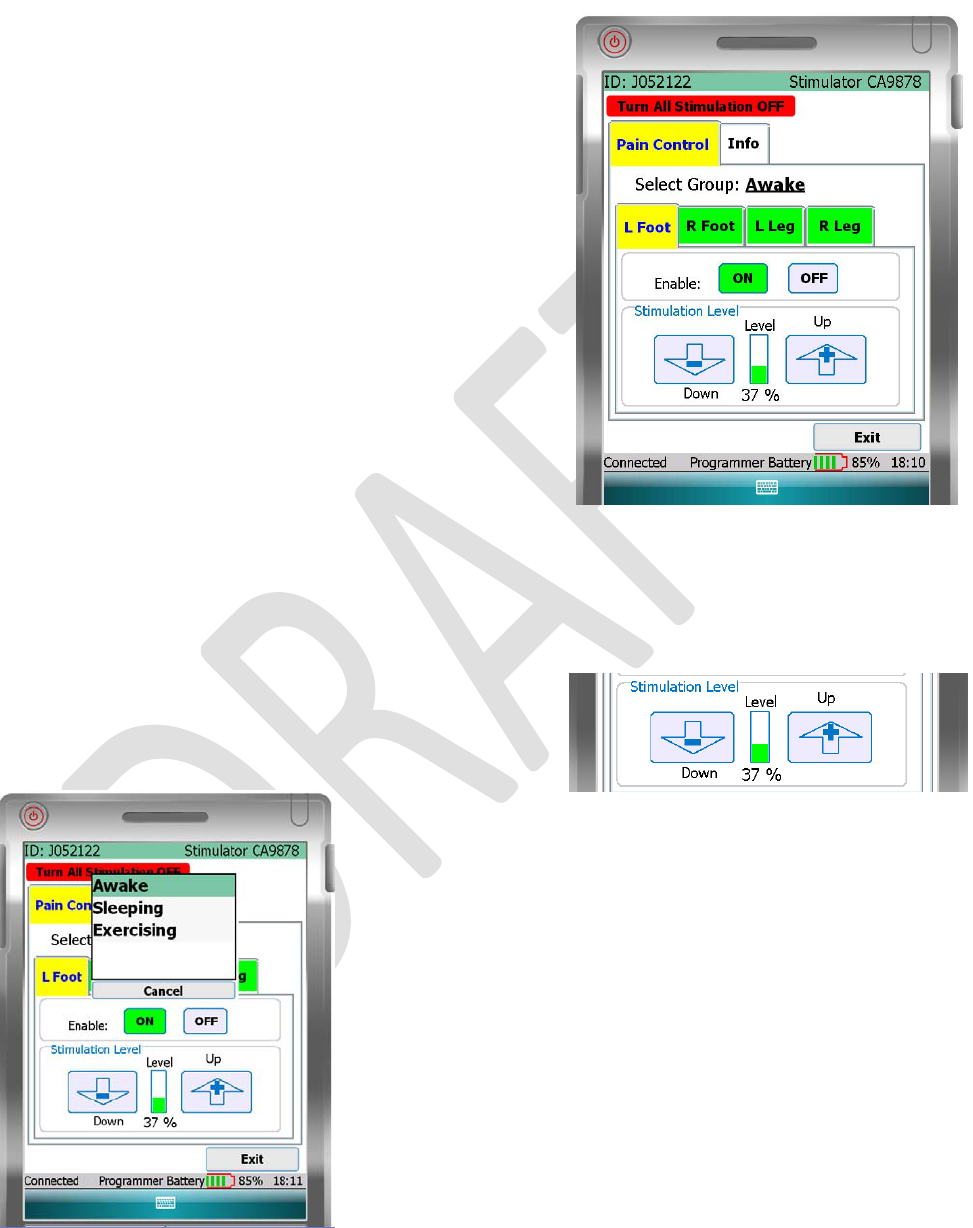
SELECTGROUP
The“SelectGroup”buttonislocatedinthecenterofthePainControl
screen.Press“SelectGroup”todisplayadropdownmenu.Thedrop
downmenuhasuptofourgroupsdefinedbyyourdoctor.Whenyou
selectagroupname,thestimulatordeviceswitchessettingstothe
newgroup.
16
PROGRAMMERSTATUSBAR
TheProgrammerStatusBarislocatedatthebottomofthePatientProgrammerscreen.Thestatusbar
showstheProgrammer‐stimulatorconnectionstatus,thebatterychargelevelandthetime.
• Programmer‐StimulatorConnectionStatus:Shows“Connecting”whenthePatientProgrammeris
tryingtoconnecttothestimulatordevice;shows“Connected”whenthePatientProgrammeris
connectedtothestimulatordevice;andshows“Disconnected”whenthePatientProgrammeris
disconnectedfromthestimulatordevice.
• ProgrammerBatteryLevel:ShowsthePatientProgrammerbatterychargelevel.
• ProgrammerClock:Showsthetime.Seethe“MainMenu”sectioninthisUserManualformoredetail.
ADJUSTINGYOURSTIMULATORDEVICESETTINGS
YoucanadjustyourstimulatordevicesettingsfromthePainControlscreen.StimulationcanbeturnedON
orOFFforuptofourregionsofyourbody.Youcanalsoadjustthestimulationlevelforanyofthoseregions.
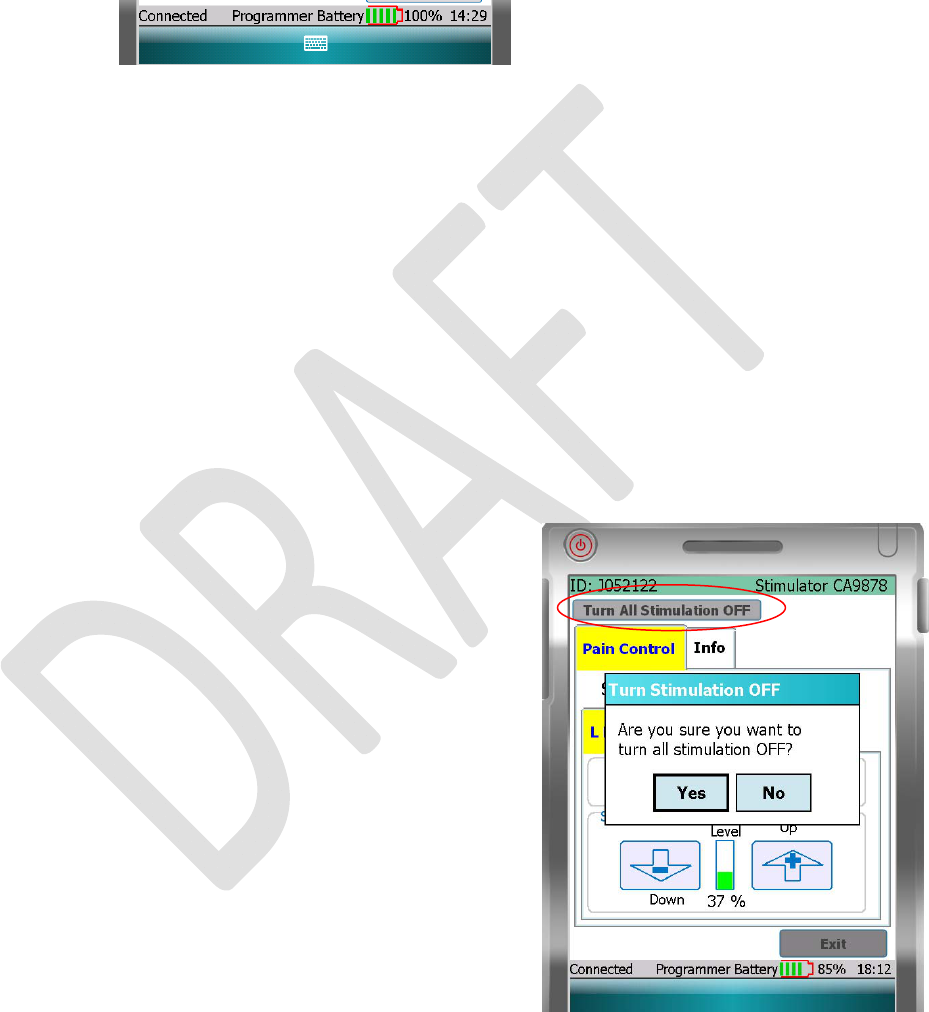
TURNINGOFFSTIMULATION
• Pressthe“TurnAllStimulationOFF”buttontostopall
stimulationtherapy.Aconfirmationwindowappears,asking
doyouwanttoturnOFFallstimulation.
NOTE:AfterturningOFFallstimulation,youcanrestore
stimulationtherapyforeachofthebodyregionsindividually.
Seethe“TurnStimulationONorOFFforaBodyRegion”
sectionbelow.
17
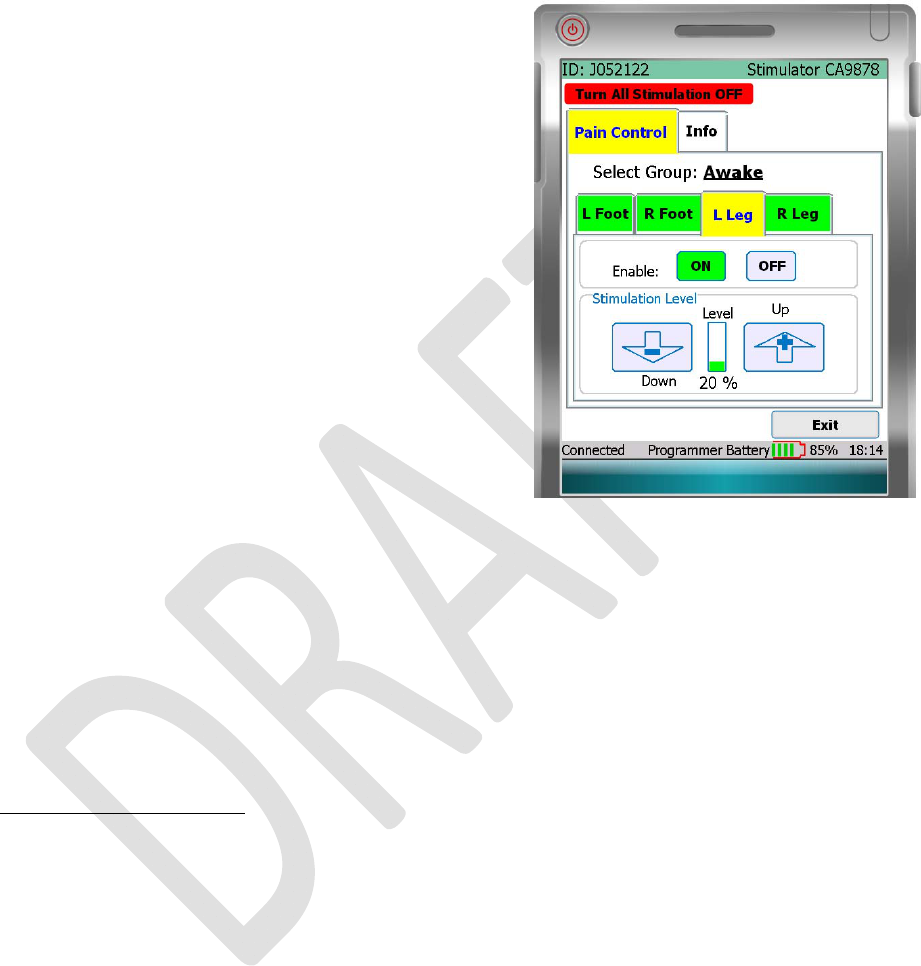
PAINCONTROLTAB
Selectthe“PainControl”tablocatedatthetopofthePain
Controlscreen.Fromthe“PainControl”tab,youcanturn
stimulationONorOFFforeachbodyregion.Youcanalso
adjustthestimulationlevelforeachbodyregion.
TURNSTIMULATIONONOROFFFORABODYREGION
YourPatientProgrammershowsthenamesofonetofour
bodyregionsinwhichyourleadshavebeenplaced.Toturn
stimulationONorOFFforabodyregion:
• Selectthebodyregionbypressingthedesiredtab.
• Pressthe“OFF”buttontostopstimulationtothat
region.WhenstimulationisOFF,the“OFF”buttonis
black.
• Pressthe“ON”buttontostartstimulationtothatregion.
WhenstimulationisON,the“ON”buttonisgreen.
ADJUSTSTIMULATIONLEVELFORABODYREGION
VerifythatyouhaveselectedthecorrectbodyregiontabonthePainControlscreen.
• Pressthe“Down”buttontodecreasethestimulationlevel.
• Pressthe“Up”buttontoincreasethestimulationlevel.
StimulationLevelIndicator:
Thestimulationlevelindicatorislocatedbetweenthe“Up”and“Down”buttons.Theindicatormoves
upordownasyouadjustthestimulationlevelfortheselectedbodyregion.Theindicatorshowsthe
currentstimulationlevelascomparedtothemaximumsetbyyourdoctor.
NOTE:Theindicatorbarisgreenwhenyouhavereachedthemaximumstimulationlevel.
18
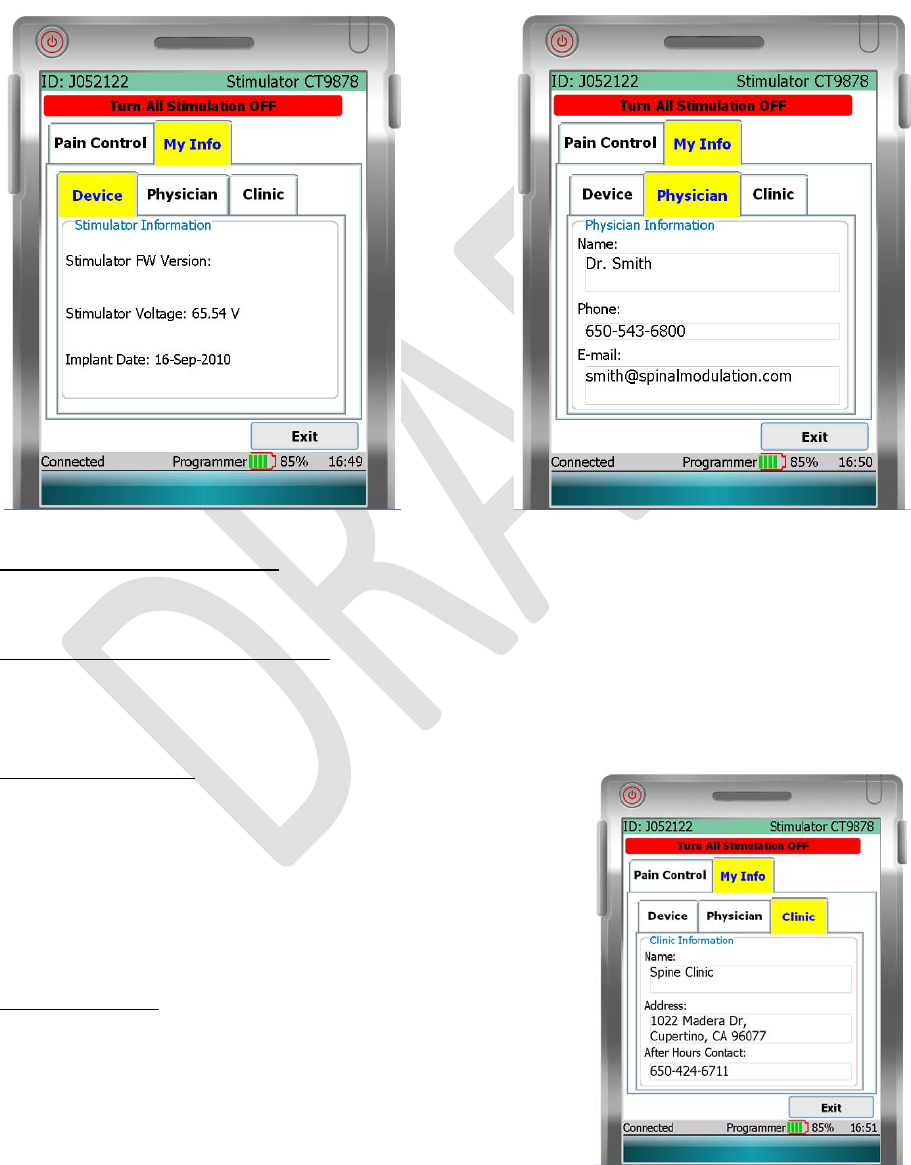
INFOTAB
The“Info”(Information)tabcontainsthreetabs,the“Device”tab,the“Physician”tab,andthe“Clinic”tab.
DEVICETABANDPHYSICIANTAB
The“Device”taband“Physician”tabshowthefollowinginformation:
StimulatorVoltageInformation
NOTE:INSbatteryinformation(doesnotpertaintoexternalTNSdevice).
StimulatorIdentificationInformation
• StimulatorDeviceFirmwareVersion.
• DateINSdevicewasimplanted(doesnotpertaintoexternalTNSdevice).
PhysicianInformation
• Yourdoctor’sname.
• Yourdoctor’scontactphonenumber.
• Yourdoctor’semailcontact.
CLINICTAB
The“Clinic”tabshowsthefollowinginformation:
ClinicInformation
• Yourclinic’sname.
• Yourclinic’saddress.
• AfterHoursContact:Aphonenumbertocontactsomeone
incaseofanemergency.
19
USINGYOURTNSDEVICE
ToconnectyourProgrammertoyourTNS,pushthe
“Connect”buttononthePatientProgrammer.Movethe
PatientProgrammermagnetovertheTNSinacircular
motion.Thiswillallowyoutoadjuststimulationsettings
usingthePatientProgrammer.Toquicklyturnstimulation
off,presstheredbuttonontheTNSformorethan2
secondsorpushthe“TurnallStimulationOFF”buttonon
theProgrammerscreen.Toenablestimulationafter
pressingeitherbutton,youmustconnectwithyourPatient
Programmerandturnthestimulationbackon.
USINGYOURINSDEVICE
ToconnectyourProgrammertoyourINS,push
the“Connect”buttononthePatient
Programmer.MovethePatientProgrammer
magnetovertheimplantlocationinacircular
motion.Thiswillallowyoutoadjuststimulation
settingsusingthePatientProgrammer.
YourSpinalModulationNeurostimulatorSystemcomplieswiththefollowingInternationalStandards
• IEC60601‐1:2005
• IEC60601‐1‐11:2010
• IEC60601‐1‐2:2007
• ISO14708‐1:2000
• ISO14708‐3:2008
AUTHORIZED EUROPEAN REPRESENTATIVE
MediTechStrategicConsultantsB.V.
Maastrichterlaan127‐129
6291ENVaals,Netherlands
COMPANY CONTACT INFORMATION
SpinalModulation,Inc.
1135O’BrienDrive
MenloPark,CA94025
U.S.A.
Telephone:(650)543‐6800(24hoursupportline)
Fax:(650)327‐2336
Email:clinicalsupport@spinalmodulation.com
20
GUIDANCE AND MANUFACTURER’S DECLARATION
ELECTROMAGNETIC EMISSIONS
The Spinal Modulation Neurostimulator System is intended for use in the electromagnetic environment specified
below. The customer or the user of the Spinal Modulation Neurostimulator System should assure that it is used
in such an environment.
Emissions test Compliance Electromagnetic environment – guidance
RF Emissions 1 Group 2 The Spinal Modulation Neurostimulator System must emit
electromagnetic energy in order to perform its intended function.
Nearby electronic equipment may be affected.
RF emissions
CISPR 11 Class B
Harmonic emissions
IEC 61000-3-2 Class B
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Complies
The Spinal Modulation Neurostimulator System is suitable for use in
all establishments, including domestic establishments and those
directly connected to the public low voltage power supply network
that supplies buildings used for domestic purposes.
CISPR 14-1 Complies The Patient Programmer is not intended to be connected to other
equipment except the Model 3400 Programmer Charger
21
Guidance and Manufacturer’s Declaration
ELECTROMAGNETIC IMMUNITY
The Spinal Modulation Neurostimulator System is intended for use in the electromagnetic environment specified
below. The customer or the user of the Spinal Modulation Neurostimulator System should assure that it is used
in such an environment.
Immunity IEC 60601
Test Level Compliance
Level Electromagnetic environment
guidance
Electrostatic discharge
(ESD) IEC 61000-4-2 ± 6 kV
contact
± 8 kV air
Floors should be wood, concrete or
ceramic tile. If floors are covered
with synthetic material, the relative
humidity should be at least 30 %.
Electrical fast
transient/burst
IEC 61000-4-4
± 2 kV for power supply lines
±1kVforinput/outputlines
Pass Mains power quality should be that
of a typical commercial or home
environment
Surge
IEC 61000-4-5 ± 1 kV line(s) to line(s)
± 2 kV line(s) to earth
Mains power quality should be that
of a typical commercial or home
environment
Voltage dips, short
interruptions and
voltage variations on
power supply
input lines
IEC 61000-4-11
<5% UT (>95% dip in UT)
for 0.5 cycle
40% UT (60% dip in UT)
for 5 cycles
70% UT (30% dip in UT)
for 25 cycles
<5% UT (>95% dip in UT)
for 5 s
NOTE UT is the a.c. mains voltage
prior to application of the test level.
Mains power quality should be that
of a typical commercial or home
environment
Power frequency
(50/60 Hz) magnetic
field
IEC 61000-4-8
3 A/m Power frequency magnetic fields
should be at levels characteristic of a
typical location in a typical
commercial, hospital, or home
environment.
22
Guidance and Manufacturer’s Declaration
ELECTROMAGNETIC IMMUNITY
The Spinal Modulation Neurostimulation System is intended for use in the electromagnetic environment specified
below. The customer or the user of the Spinal Modulation Neurostimulation System should assure that it is used in
such an environment
Immunity test IEC 60601 TEST
LEVEL Compliance
level Electromagnetic environment guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2,5 GHz
3 V
3 V/m
Portable and mobile RF communications
equipment should be used no closer to any
part of Spinal Modulation Neurostimulation
System, than 0.2 meter, based on
transmitters of 80 MHz to 2.5 GHz.
Interference may occur in the vicinity of
equipment marked with the following
symbol:
Recommended separation distances between portable and mobile RF communications equipment
and the Spinal Modulation Neurostimulation System
The Spinal Modulation Neurostimulation System is intended for use in an electromagnetic environment in which
radiated RF disturbances are controlled. The customer or the user of the Spinal Modulation Neurostimulation
System can help prevent electromagnetic interference by maintaining a minimum distance between portable and
mobile RF communications equipment (transmitters) and the System
Separation distance according to frequency of transmitter
m
Rated maximum
output power of
transmitter
W 150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5 GHz
0.01 0.12m 0.12m 0.23m
0.1 0.37m 0.37m 0.74m
1 1.17m 1.17m 2.33m
10 3.70m 3.70m 7.37m
100 11.70m 11.70m 23.30m
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption
and reflection from structures, objects and people.
23
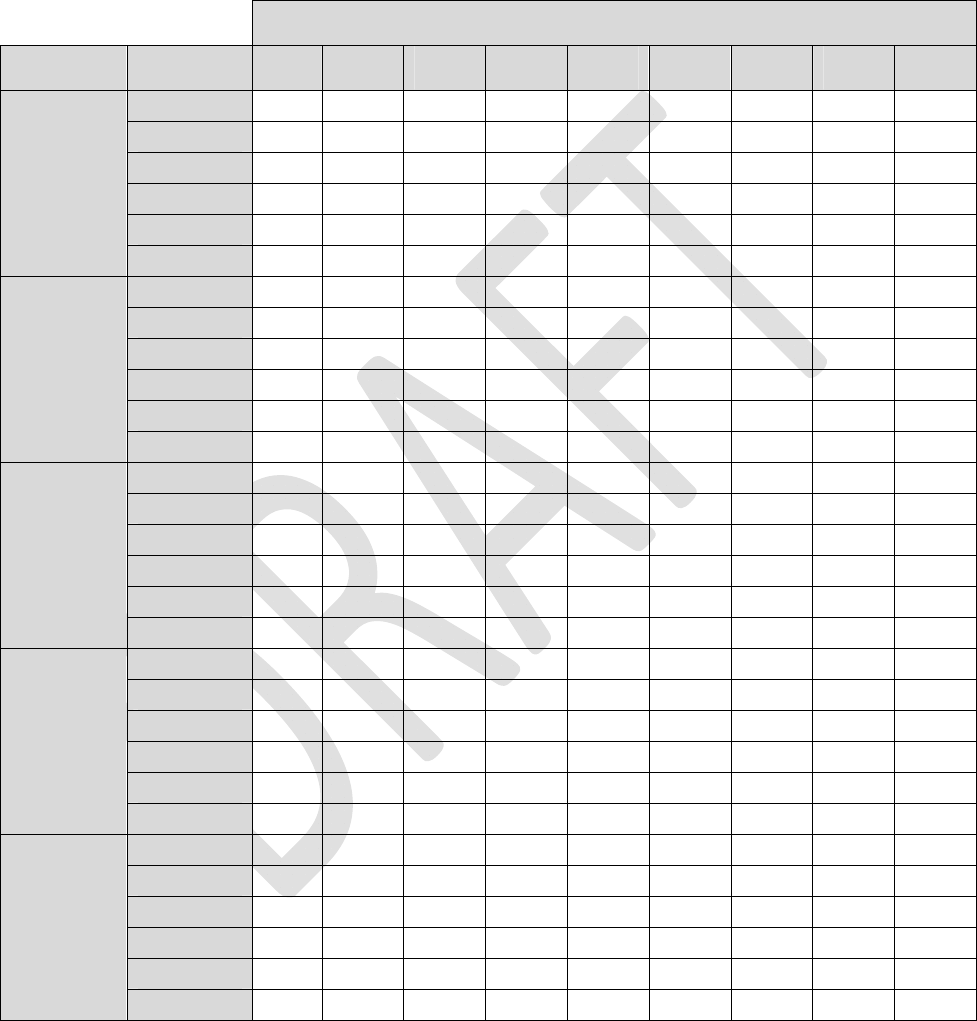
Appendix I: INS Battery Life
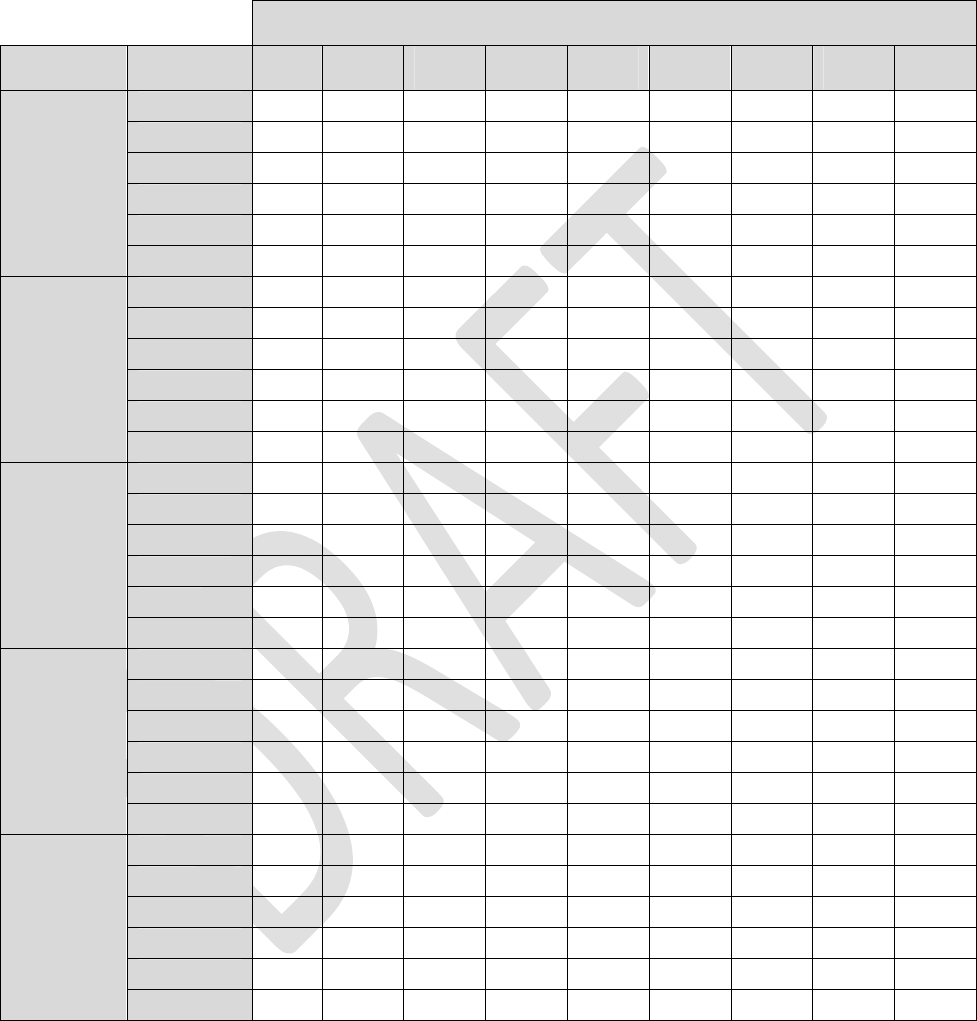
ThefollowingfourtablesestimateINSbatterylifeunderthegivenloadimpedanceconditions.
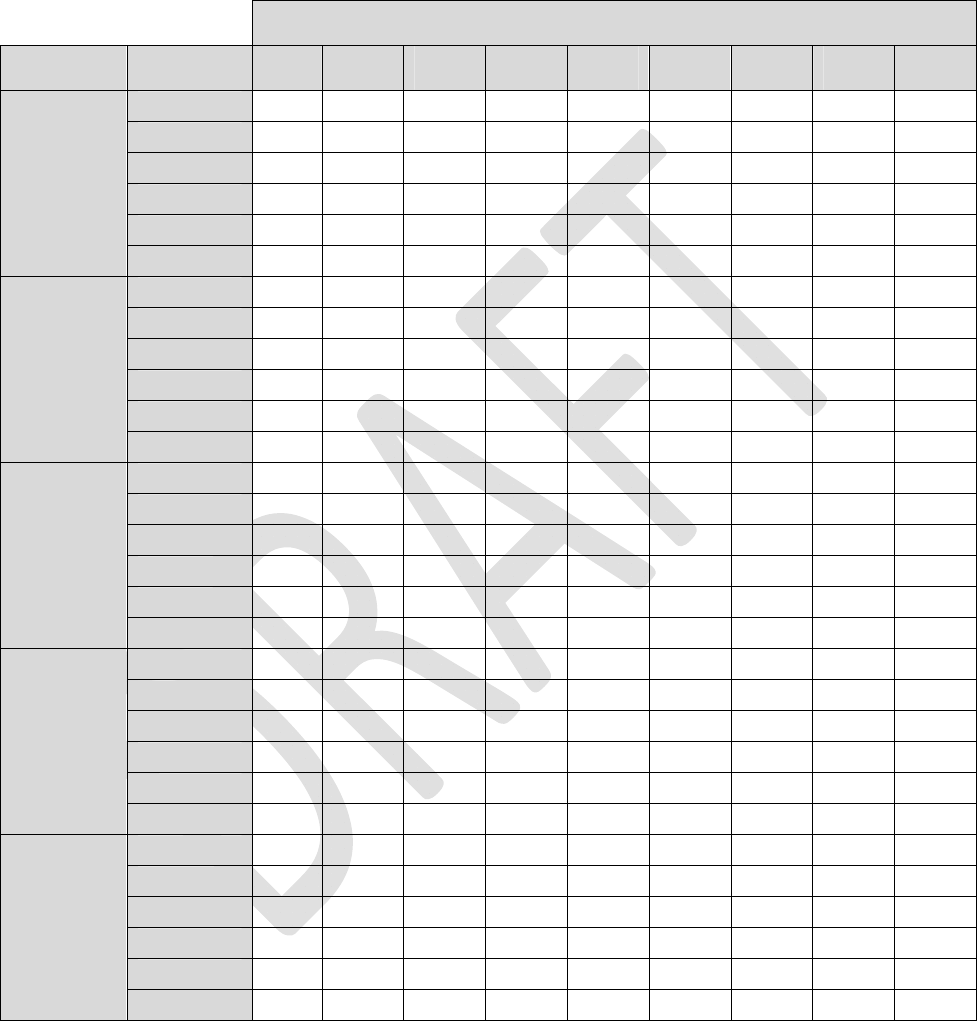
Tables1through4listestimatedlongevitybasedon1‐leadstimulation,active24hoursaday.Toestimate
longevityformultipleactiveleadsandtoaccountfordailyusage,usethefollowingformula:
Where,
C2 = Longevity for Lead 2 Settings Daily Usage Lead 2
-35
3014
And,
DailyUsageLead1,2,3and4arefractionalvaluesequaltothenumberofhoursstimulationisenabledfor
eachleaddailydividedby24hours.
Note:DonotentervaluesforC1,C2,C3orC4forcorrespondingleadsthatareinactive.
Example:
Estimatelongevityfor2leadstimulationwithlead1settingsof0.8mA,40Hz,200µsacross600ohms
continuouslystimulating,andlead2settingsof1.0mA,60Hz,400µsacross1Kohmstimulatingfor16hours
perday.
FromTable1(600ohms),longevityforlead1settings=58.9months
FromTable2(1Kohm),longevityforlead2settings=34.1months
CalculatedC1=(3014/58.9–35)*(24/24)=16.2
CalculatedC2=(3014/34.1–35)*(16/24)=35.6
Estimatedlongevityforthisexample=3014/(16.2+35.6+35)=34.7months
24
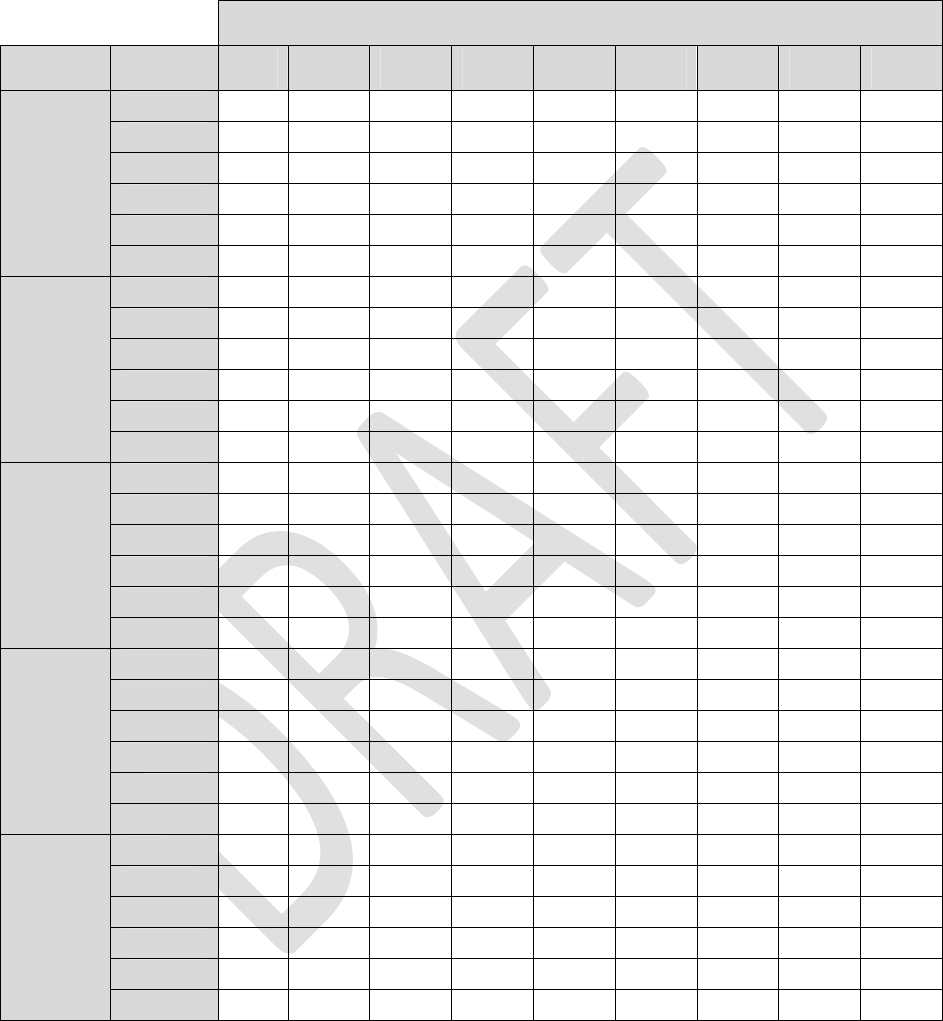
Table1:LoadImpedance=600ohms
Alllongevityvaluesareinmonths
Pulsewidth
AmplitudeFrequency40uS100uS 200uS 300uS 400uS 500uS600uS700uS 720uS
4Hz83.983.883.683.483.283.082.882.782.6
20Hz81.580.980.179.278.377.576.775.975.8
40Hz78.677.676.074.573.071.670.268.968.6
60Hz76.074.672.470.368.366.564.763.162.7
80Hz73.571.869.166.664.262.160.058.157.8
0.1mA
100Hz71.269.266.163.260.658.256.053.953.5
4Hz83.883.683.282.882.582.181.781.481.3
20Hz81.180.178.376.775.173.672.270.870.5
40Hz78.076.073.070.267.665.263.060.960.5
60Hz75.172.468.364.761.558.555.953.453.0
80Hz72.469.164.260.056.453.150.247.647.1
0.2mA
100Hz69.866.160.656.052.048.645.642.942.4
4Hz83.783.282.581.781.080.379.678.978.8
20Hz80.478.375.172.269.466.964.662.461.9
40Hz76.773.067.663.058.955.452.249.448.9
60Hz73.368.361.555.951.247.243.840.940.4
80Hz70.164.256.450.245.241.237.834.934.4
0.4mA
100Hz67.360.652.045.640.536.533.230.429.9
4Hz83.582.881.780.779.678.677.676.676.4
20Hz79.776.772.268.264.661.358.455.755.2
40Hz75.470.263.057.152.248.144.641.641.0
60Hz71.564.755.949.143.839.636.133.232.6
80Hz68.160.050.243.137.833.630.327.627.1
0.6mA
100Hz64.956.045.638.433.229.226.123.623.1
4Hz83.482.581.079.678.276.975.774.474.2
20Hz79.075.169.464.660.356.653.350.449.8
40Hz74.267.658.952.246.942.538.935.935.3
60Hz69.961.551.243.838.334.130.727.927.4
80Hz66.156.445.237.832.428.425.322.822.3
0.8mA
100Hz62.752.040.533.228.124.421.519.318.9
25
Table1:LoadImpedance=600ohms(Continued)
Alllongevityvaluesareinmonths
Pulsewidth
40uS100uS 200uS 300uS 400uS 500uS 600uS700uS 720uS
4Hz83.282.180.378.676.975.473.872.472.1
20Hz78.373.666.961.356.652.549.046.045.4
40Hz73.065.255.448.142.538.134.531.631.0
60Hz68.358.547.239.634.129.926.624.023.6
80Hz64.253.141.233.628.424.621.719.419.0
1.0mA
100Hz60.648.636.529.224.420.918.316.315.9
4Hz82.881.278.676.173.871.769.667.767.3
20Hz76.770.161.354.549.044.640.837.737.1
40Hz70.259.948.140.234.530.326.924.323.8
60Hz64.752.339.631.926.622.920.117.917.5
80Hz60.046.433.626.421.718.416.014.213.8
1.5mA
100Hz56.041.729.222.518.315.413.311.711.4
4Hz82.580.376.973.871.068.365.963.663.2
20Hz75.166.956.649.043.338.735.032.031.4
40Hz67.655.442.534.529.125.122.119.719.3
60Hz61.547.234.126.621.918.616.114.213.9
80Hz56.441.228.421.717.514.712.711.110.9
2.0mA
100Hz52.036.524.418.314.612.210.59.28.9
4Hz56.653.849.746.243.140.538.136.035.6
20Hz46.338.229.624.120.417.615.513.913.6
40Hz37.728.019.615.112.310.38.97.87.7
60Hz31.822.114.711.08.87.36.35.55.3
80Hz27.518.311.78.66.85.64.84.24.1
4.0mA
100Hz24.315.69.87.15.64.63.93.43.3
4Hz52.647.440.835.831.828.726.124.023.6
20Hz35.325.917.913.711.19.38.07.06.9
40Hz25.016.510.57.76.15.04.33.73.6
60Hz19.412.17.45.44.23.52.92.52.5
80Hz15.89.65.84.13.22.62.21.91.9
6.0mA
100Hz13.37.94.73.32.62.11.81.61.5
26
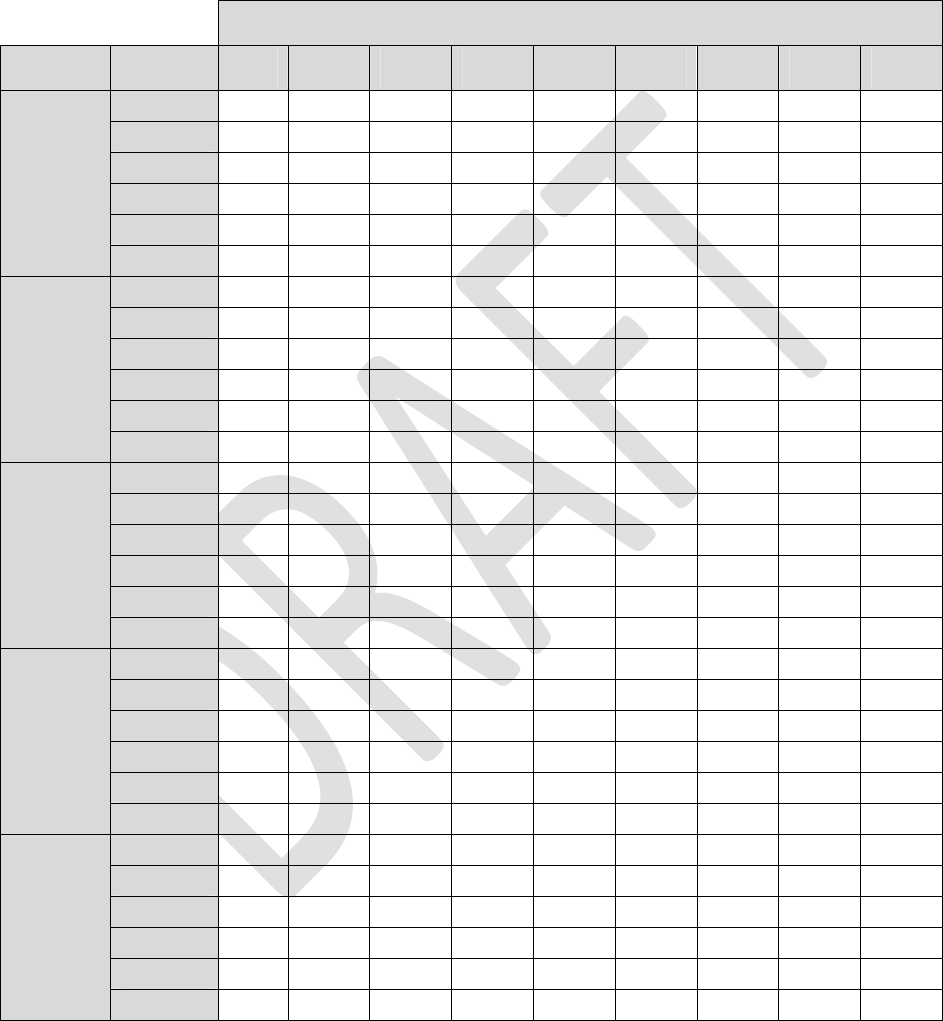
Table2:LoadImpedance=1Kohm
Alllongevityvaluesareinmonths
Pulsewidth
AmplitudeFrequency40uS100uS 200uS 300uS 400uS 500uS600uS700uS 720uS
4Hz83.983.883.683.483.283.082.882.782.6
20Hz81.580.980.179.278.377.576.775.975.8
40Hz78.677.676.074.573.071.670.268.968.6
60Hz76.074.672.470.368.366.564.763.162.7
80Hz73.571.869.166.664.262.160.058.157.8
0.1mA
100Hz71.269.266.163.260.658.256.053.953.5
4Hz83.883.683.282.882.582.181.781.481.3
20Hz81.180.178.376.775.173.672.270.870.5
40Hz78.076.073.070.267.665.263.060.960.5
60Hz75.172.468.364.761.558.555.953.453.0
80Hz72.469.164.260.056.453.150.247.647.1
0.2mA
100Hz69.866.160.656.052.048.645.642.942.4
4Hz83.783.282.581.781.080.379.678.978.8
20Hz80.478.375.172.269.466.964.662.461.9
40Hz76.773.067.663.058.955.452.249.448.9
60Hz73.368.361.555.951.247.243.840.940.4
80Hz70.164.256.450.245.241.237.834.934.4
0.4mA
100Hz67.360.652.045.640.536.533.230.429.9
4Hz83.582.881.780.779.678.677.676.676.4
20Hz79.776.772.268.264.661.358.455.755.2
40Hz75.470.263.057.152.248.144.641.641.0
60Hz71.564.755.949.143.839.636.133.232.6
80Hz68.160.050.243.137.833.630.327.627.1
0.6mA
100Hz64.956.045.638.433.229.226.123.623.1
4Hz83.482.581.079.678.276.975.774.474.2
20Hz79.075.169.464.660.356.653.350.449.8
40Hz74.267.658.952.246.942.538.935.935.3
60Hz69.961.551.243.838.334.130.727.927.4
80Hz66.156.445.237.832.428.425.322.822.3
0.8mA
100Hz62.752.040.533.228.124.421.519.318.9
27
Table2:LoadImpedance=1Kohm(Continued)
Alllongevityvaluesareinmonths
Pulsewidth
40uS100uS 200uS 300uS 400uS 500uS 600uS700uS 720uS
4Hz83.282.180.378.676.975.473.872.472.1
20Hz78.373.666.961.356.652.549.046.045.4
40Hz73.065.255.448.142.538.134.531.631.0
60Hz68.358.547.239.634.129.926.624.023.6
80Hz64.253.141.233.628.424.621.719.419.0
1.0mA
100Hz60.648.636.529.224.420.918.316.315.9
4Hz82.881.278.676.173.871.769.667.767.3
20Hz76.770.161.354.549.044.640.837.737.1
40Hz70.259.948.140.234.530.326.924.323.8
60Hz64.752.339.631.926.622.920.117.917.5
80Hz60.046.433.626.421.718.416.014.213.8
1.5mA
100Hz56.041.729.222.518.315.413.311.711.4
4Hz58.457.255.153.351.549.948.346.946.6
20Hz53.148.241.836.933.029.827.325.124.7
40Hz47.740.332.126.622.819.917.615.915.5
60Hz43.234.626.020.817.414.913.011.611.3
80Hz39.630.421.917.114.011.910.39.18.9
2.0mA
100Hz36.527.018.914.511.89.98.67.57.4
4Hz53.049.143.639.235.732.730.228.027.6
20Hz36.328.420.916.513.611.610.19.08.8
40Hz26.118.612.69.67.76.45.54.84.7
60Hz20.313.99.16.75.34.43.83.33.2
80Hz16.711.07.15.24.13.42.92.52.5
4.0mA
100Hz14.19.25.84.23.32.72.32.02.0
4Hz51.446.540.235.431.628.626.124.023.6
20Hz32.724.517.413.410.99.28.07.06.9
40Hz22.415.410.17.66.05.04.33.73.7
60Hz17.111.37.25.34.23.42.92.52.5
80Hz13.88.95.54.03.22.62.21.91.9
6.0mA
100Hz11.67.34.53.32.62.11.81.61.5
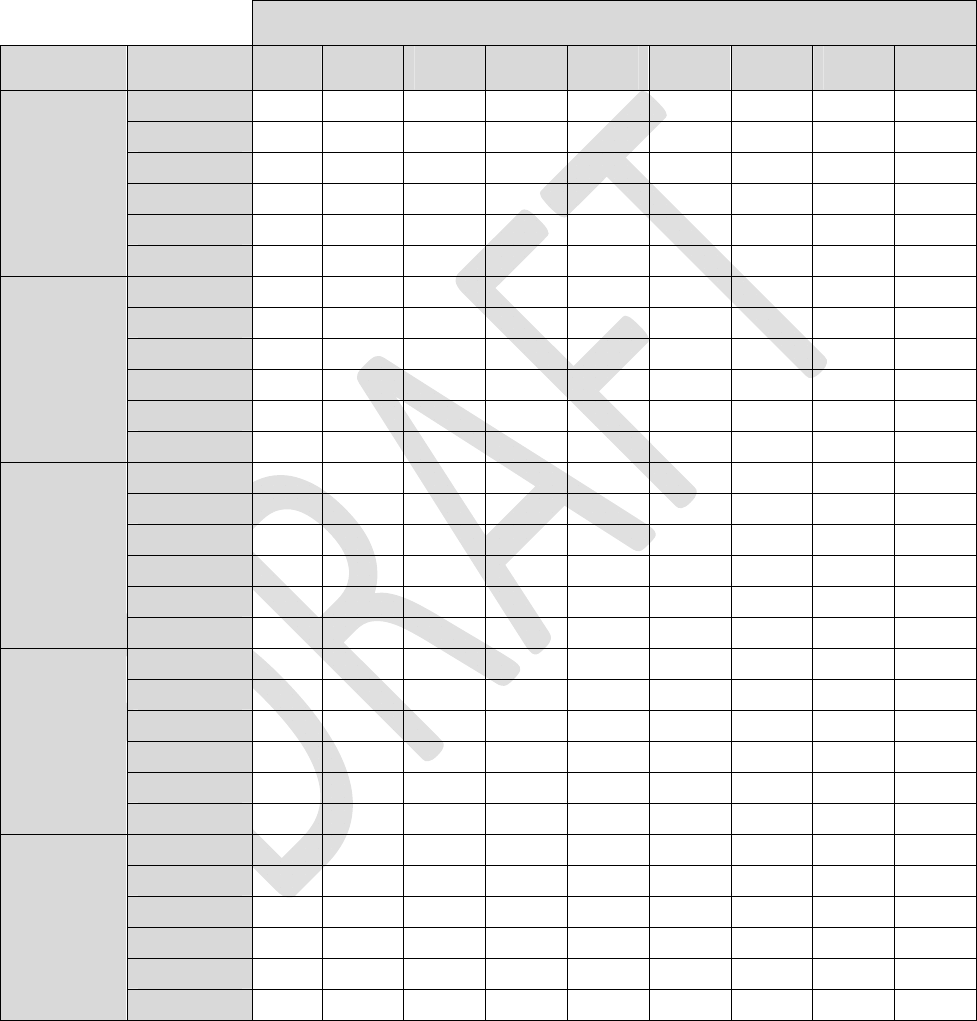
28
Table3:LoadImpedance=1.5Kohm
Alllongevityvaluesareinmonths
Pulsewidth
AmplitudeFrequency40uS100uS 200uS 300uS 400uS 500uS600uS700uS 720uS
4Hz83.983.883.683.483.283.082.882.782.6
20Hz81.580.980.179.278.377.576.775.975.8
40Hz78.677.676.074.573.071.670.268.968.6
60Hz76.074.672.470.368.366.564.763.162.7
80Hz73.571.869.166.664.262.160.058.157.8
0.1mA
100Hz71.269.266.163.260.658.256.053.953.5
4Hz83.883.683.282.882.582.181.781.481.3
20Hz81.180.178.376.775.173.672.270.870.5
40Hz78.076.073.070.267.665.263.060.960.5
60Hz75.172.468.364.761.558.555.953.453.0
80Hz72.469.164.260.056.453.150.247.647.1
0.2mA
100Hz69.866.160.656.052.048.645.642.942.4
4Hz83.783.282.581.781.080.379.678.978.8
20Hz80.478.375.172.269.466.964.662.461.9
40Hz76.773.067.663.058.955.452.249.448.9
60Hz73.368.361.555.951.247.243.840.940.4
80Hz70.164.256.450.245.241.237.834.934.4
0.4mA
100Hz67.360.652.045.640.536.533.230.429.9
4Hz83.582.881.780.779.678.677.676.676.4
20Hz79.776.772.268.264.661.358.455.755.2
40Hz75.470.263.057.152.248.144.641.641.0
60Hz71.564.755.949.143.839.636.133.232.6
80Hz68.160.050.243.137.833.630.327.627.1
0.6mA
100Hz64.956.045.638.433.229.226.123.623.1
4Hz83.482.581.079.678.276.975.774.474.2
20Hz79.075.169.464.660.356.653.350.449.8
40Hz74.267.658.952.246.942.538.935.935.3
60Hz69.961.551.243.838.334.130.727.927.4
80Hz66.156.445.237.832.428.425.322.822.3
0.8mA
100Hz62.752.040.533.228.124.421.519.318.9
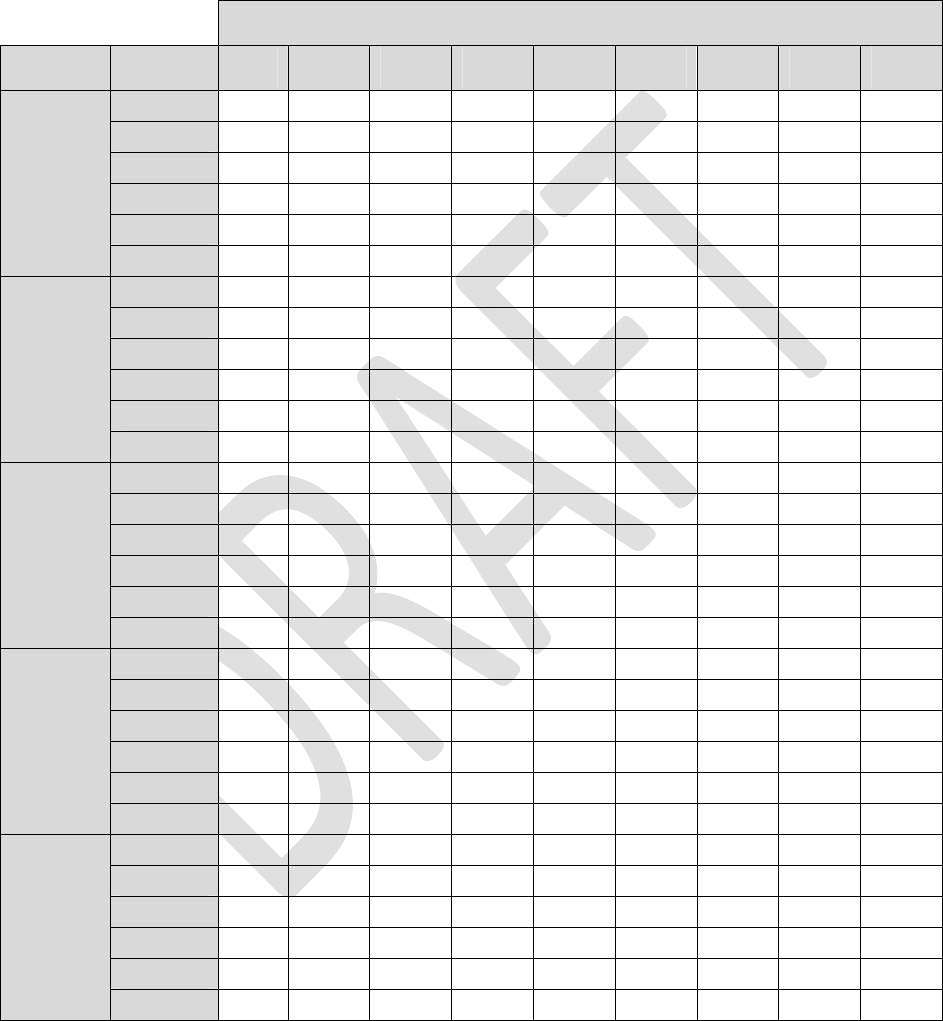
29
Table3:LoadImpedance=1.5Kohm(Continued)
Alllongevityvaluesareinmonths
Pulsewidth
40uS100uS 200uS 300uS 400uS 500uS 600uS700uS 720uS
4Hz83.282.180.378.676.975.473.872.472.1
20Hz78.373.666.961.356.652.549.046.045.4
40Hz73.065.255.448.142.538.134.531.631.0
60Hz68.358.547.239.634.129.926.624.023.6
80Hz64.253.141.233.628.424.621.719.419.0
1.0mA
100Hz60.648.636.529.224.420.918.316.315.9
4Hz58.257.155.453.852.350.849.548.247.9
20Hz52.047.942.538.134.631.629.127.026.6
40Hz45.940.032.927.924.321.519.317.417.1
60Hz41.134.226.822.118.716.314.412.912.6
80Hz37.230.022.718.215.213.111.510.210.0
1.5mA
100Hz33.926.619.615.512.810.99.58.58.3
4Hz56.454.651.949.547.245.243.341.641.3
20Hz45.740.433.829.125.622.820.518.718.4
40Hz36.930.423.619.216.214.112.411.110.9
60Hz30.924.418.114.411.910.28.97.97.7
80Hz26.620.414.711.59.48.06.96.16.0
2.0mA
100Hz23.417.512.39.57.86.55.75.04.9
4Hz52.649.144.240.236.934.131.629.529.1
20Hz35.328.521.617.414.512.510.99.79.5
40Hz25.018.713.210.18.37.06.05.35.2
60Hz19.313.99.57.25.84.84.23.63.6
80Hz15.811.17.45.54.43.73.22.82.7
4.0mA
100Hz13.39.26.14.53.63.02.62.22.2
4Hz52.649.144.240.236.934.131.629.529.1
20Hz35.328.521.617.414.512.510.99.79.5
40Hz25.018.713.210.18.37.06.05.35.2
60Hz19.313.99.57.25.84.84.23.63.6
80Hz15.811.17.45.54.43.73.22.82.7
6.0mA
100Hz13.39.26.14.53.63.02.62.22.2
30
Table4:LoadImpedance=2Kohm
Alllongevityvaluesareinmonths
Pulsewidth
AmplitudeFrequency40uS100uS 200uS 300uS 400uS 500uS600uS700uS 720uS
4Hz83.983.883.683.483.283.082.882.782.6
20Hz81.580.980.179.278.377.576.775.975.8
40Hz78.677.676.074.573.071.670.268.968.6
60Hz76.074.672.470.368.366.564.763.162.7
80Hz73.571.869.166.664.262.160.058.157.8
0.1mA
100Hz71.269.266.163.260.658.256.053.953.5
4Hz83.883.683.282.882.582.181.781.481.3
20Hz81.180.178.376.775.173.672.270.870.5
40Hz78.076.073.070.267.665.263.060.960.5
60Hz75.172.468.364.761.558.555.953.453.0
80Hz72.469.164.260.056.453.150.247.647.1
0.2mA
100Hz69.866.160.656.052.048.645.642.942.4
4Hz83.783.282.581.781.080.379.678.978.8
20Hz80.478.375.172.269.466.964.662.461.9
40Hz76.773.067.663.058.955.452.249.448.9
60Hz73.368.361.555.951.247.243.840.940.4
80Hz70.164.256.450.245.241.237.834.934.4
0.4mA
100Hz67.360.652.045.640.536.533.230.429.9
4Hz83.582.881.780.779.678.677.676.676.4
20Hz79.776.772.268.264.661.358.455.755.2
40Hz75.470.263.057.152.248.144.641.641.0
60Hz71.564.755.949.143.839.636.133.232.6
80Hz68.160.050.243.137.833.630.327.627.1
0.6mA
100Hz64.956.045.638.433.229.226.123.623.1
4Hz83.482.581.079.678.276.975.774.474.2
20Hz79.075.169.464.660.356.653.350.449.8
40Hz74.267.658.952.246.942.538.935.935.3
60Hz69.961.551.243.838.334.130.727.927.4
80Hz66.156.445.237.832.428.425.322.822.3
0.8mA
100Hz62.752.040.533.228.124.421.519.318.9
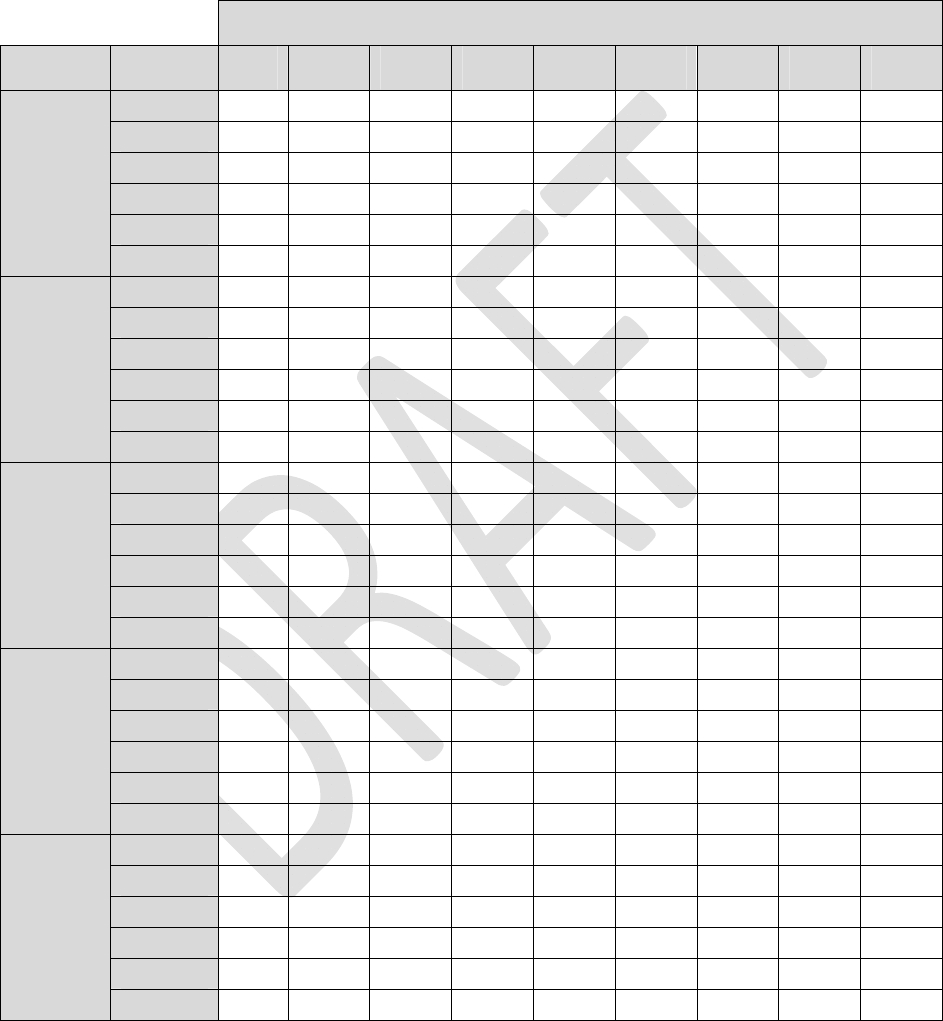
31
Table4:LoadImpedance=2Kohm(Continued)
Alllongevityvaluesareinmonths
Pulsewidth
40uS100uS 200uS 300uS 400uS 500uS 600uS700uS 720uS
4Hz58.958.257.256.155.154.253.352.452.2
20Hz55.052.248.244.841.839.236.934.834.4
40Hz50.746.340.335.732.129.126.624.524.2
60Hz47.141.534.629.726.023.120.818.918.6
80Hz44.037.730.425.421.919.217.115.415.1
1.0mA
100Hz41.234.527.022.218.916.414.513.012.7
4Hz56.755.453.251.349.547.846.244.744.4
20Hz46.742.436.832.529.126.424.122.221.8
40Hz38.232.826.622.319.216.915.113.613.3
60Hz32.426.820.817.014.412.411.09.89.6
80Hz28.122.617.113.711.59.88.67.77.5
1.5mA
100Hz24.819.614.511.59.58.17.16.36.2
4Hz54.552.349.146.243.641.339.239.237.0
20Hz40.134.728.424.120.918.416.516.514.6
40Hz30.124.518.615.112.610.99.69.68.3
60Hz24.118.913.911.09.17.76.76.75.8
80Hz20.115.411.08.67.16.05.25.24.5
2.0mA
100Hz17.213.09.27.15.84.94.24.23.6
4Hz53.250.546.543.140.237.735.433.433.0
20Hz36.731.024.520.317.415.113.412.111.8
40Hz26.520.915.412.210.18.77.66.76.6
60Hz20.715.711.38.87.26.15.34.64.5
80Hz17.012.68.96.85.54.74.03.63.5
4.0mA
100Hz14.410.67.35.64.53.83.32.92.8
4Hz53.250.546.543.140.237.735.433.433.0
20Hz36.731.024.520.317.415.113.412.111.8
40Hz26.520.915.412.210.18.77.66.76.6
60Hz20.715.711.38.87.26.15.34.64.5
80Hz17.012.68.96.85.54.74.03.63.5
6.0mA
100Hz14.410.67.35.64.53.83.32.92.8
32
Appendix II: Troubleshooting
Pop Up Message Possible Solution
Connection with your stimulator was lost. Please recon-
nect.
• Swipe the programmer over the stimulator to establish connection
and reconnect to the device.
• If you still cannot connect, move the programmer closer to the
stimulator. Continue to swipe the magnet over the stimulator.
• Move to another location; there may be interference in your current
location.
Unable to connect to your stimulator.
Please contact your physician during normal business
hours.
• Press “OK” and attempt to reconnect to the stimulator.
• Swipe the programmer over the stimulator to establish connection
and reconnect to the device.
• If you still cannot connect, move the programmer closer to the
stimulator and continue to swipe the magnet over the stimulator.
• Move to another location; there may be interference in your current
location.
• Contact your doctor during normal business hours if the problem
continues.
Unable to connect to your stimulator. Please try again. • Swipe the programmer over the stimulator to establish connection
and reconnect to the device.
• If you still cannot connect, move the programmer closer to the
stimulator and continue to swipe the magnet over the stimulator.
• Move to another location; there may be interference in your current
location.
Your stimulator battery is low. It will need to be replaced
soon. Please contact your physician during normal
business hours (only applies to the INS).
• Contact your doctor during normal business hours to set up an
appointment. Your stimulator has reached Elective Replacement
Interval (ERI).
Your stimulator battery needs to be replaced. Stimulation
has been turned OFF permanently.
Please contact your physician during normal business
hours (only applies to the INS).
• Contact your doctor during normal business hours to set up an
appointment. Your stimulator has reached End of Service (EOS)
and will not stimulate. It must be replaced.
Stimulation for one or more leads has been turned OFF.
Please contact your physician during normal business
hours.
• Swipe the programmer over the stimulator to establish connection
and reconnect to the device.
• Go the Pain Control screen and turn on the lead that has been
turned off.
• If you are unable to turn it back on, contact your doctor during
normal business hours.
All stimulation has been turned OFF. Please contact your
physician during normal business hours.
• Contact your doctor during normal business hours.
Stimulation has been turned OFF due to a magnet. Please
use your programmer to restore stimulation.
• Swipe the programmer over the stimulator to establish connection
and reconnect to the device.
• Go to the Pain Control screen. Turn on each lead one at a time.
You have turned all Stimulation OFF.
Please use your programmer to restore stimulation.
• You have turned off the device by pressing the switch on the TNS or
by pressing the “Turn All Stimulation OFF” button on the
programmer.
• Swipe the programmer over the stimulator to establish connection
and reconnect to the device.
• Go the Pain Control screen. Turn on the leads that have been
turned off.
Programmer battery is low. Please recharge. • The battery has reached 30% on the programmer and needs to be
recharged.