ERBE Elektromedizin VIO3 Electrosurgical Unit with WLAN User Manual OBJ DOKU 195191 001
ERBE Elektromedizin GmbH Electrosurgical Unit with WLAN OBJ DOKU 195191 001
Contents
- 1. user manual I
- 2. user manual II
- 3. User manual III
User manual III
137 / 158
14 • Installation
80114-601
03.16
WARNING
Incorrect line fuse, defective device
Risk of electric shock to the patient and medical personnel! Risk of
damage to property.
Blown line fuses may only be replaced by a competent technician.
Only replacement fuses that have the same rating as the one
specified on the unit’s rating plate may be used.
When a fuse has been changed, the function of the unit must be
verified. If the unit does not function properly or if there are any
concerns, please contact Erbe.
WARNING
Damaged device, damaged accessories, modified device, and
modified accessories
Risk of burns and injury to the patient and medical personnel! Risk
of damage to property.
Check the device and accessories for damage every time before
using them (e.g. footswitch, cords of instruments and the return
electrode, equipment cart).
You must not use damaged equipment or damaged accessories.
Replace defective accessories.
If the equipment or equipment cart is damaged, please contact
our customer service.
For your safety and that of the patient: Never attempt to perform
repairs or make modifications yourself. Any modification will in-
validate liability on the part of Erbe Elektromedizin GmbH.
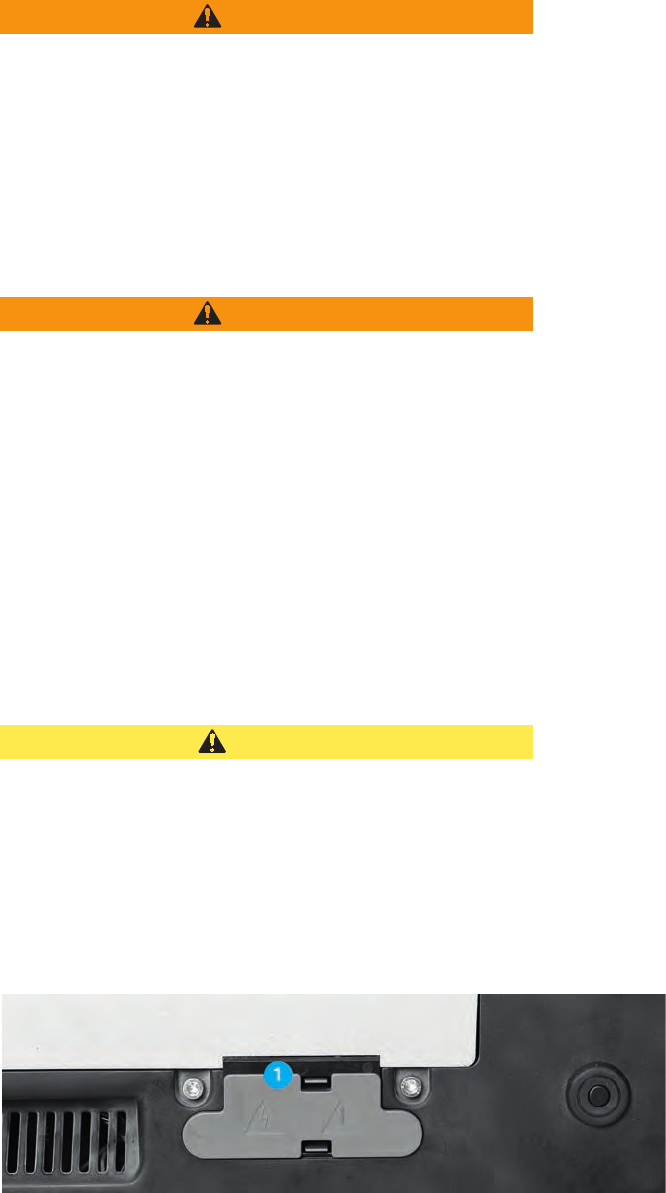
CAUTION
The interconnections of the VIO 3 carry HF voltage when they are
activated.
If you touch the interconnections during activation, you can suffer
burns.
You may only remove the cap (1) (fig. below) if you install the VIO
3 on an APC 3.
Keep the cap in a safe place. If you disconnect the VIO 3 from the
APC 3, you must replace the cap on the interconnections.
Fig. 14-1
Access to the power cord Note: Install the device such that the power cord can be pulled out without problems.
Grounding
Note:
If necessary, the equipment can be connected to the external grounding system of
the room with the grounding pin on the back of the unit and/or Cart using a connecting
cable designed for this purpose. Affects of low frequency leakage currents due to a de-
fective grounding system within the room may be eliminated through external grounding.
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.

14 • Installation
138 / 158
80114-601
03.16
Installation of the rear of the VIO 3
Fig. 14-2
Footswitch sockets
You connect a two-pedal and a one-pedal footswitch to these sockets. The combina-
tions of two two-pedal footswitches or two one-pedal footswitches are not possible.
ECB sockets (Erbe Communication Bus)
These sockets serve to connect other units with the VIO 3.
Grounding terminal connection
If necessary, connect the grounding pin of the unit to the grounding system of the op-
erating room using a grounding cable.
Power connection
Connect the unit to a properly installed grounded power outlet. Only use the provided
power cord for this purpose. The power cord must bear the national test symbol.
Optionally, you can connect a power cord with V lock. The unit plug locks into the pow-
er connection of the VIO 3 and cannot loosen on its own.
Power fuses
The unit is protected with power fuses. If one of these power fuses has blown, the unit
may not be used on the patient again until it has been checked by a competent tech-
nician. The values of the power fuses are specified on the unit's rating plate. Only
spare fuses with these values may be used.
Footswitch sockets
Grounding terminal
Line fuses
Power connection
ECB sockets
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.
139 / 158
14 • Installation
80114-601
03.16
Installation of the VIO 3 on an overhead suspension arm system
For the installation of the VIO 3 on an overhead suspension arm system, you require
fastening set no. 20180-143. Installation instructions are included with the fastening
set. Install the VIO 3 according to the installation instructions.
Installation of the VIO 3 on an Erbe equipment cart
Please read the User Manual for the equipment cart concerned. There you will find in-
structions on how to secure the unit to the equipment cart.
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.
14 • Installation
140 / 158
80114-601
03.16
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.
141 / 158
15 • Cleaning and Disinfection
80114-601
03.16
Chapter 15
Cleaning and Disinfection
Safety Instructions
WARNING
Connection of unit / equipment cart and power supply during
cleaning and disinfection
Risk of electric shock to the medical personnel!
Switch off the device. Unplug the power cord of the device/equip-
ment cart.
WARNING
Flammable detergents and disinfectants, flammable solvents in
adhesives used on the patient and on the device / equipment cart
Risk of fire and explosion to the patient and medical personnel! Risk
of damage to property.
Use products that are not flammable.
If the use of flammable products is unavoidable, proceed as fol-
lows:
Allow the products to evaporate completely before switching on
the device.
Check whether flammable liquids have accumulated under the
patient, in body recesses such as the navel, or in body cavities
such as the vagina. Remove any liquids before performing elec-
trosurgery.
NOTICE
Penetration of liquid into the device
The housing is not absolutely watertight. If liquid penetrates, the de-
vice can sustain damage and fail.
Make sure no liquid can penetrate the device.
Do not place vessels containing liquids on top of the device.
NOTICE
Alcohol-based spray disinfectant for fast disinfection
In the case of elastic molded parts and paint surfaces, there is a risk
of formation of cracks. Propanol and ethanol will attack the surfaces.
Do not use these substances.
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.

15 • Cleaning and Disinfection
142 / 158
80114-601
03.16
NOTICE
Alternate use of disinfectant solutions based on different active
ingredients
A color reaction may occur with plastics.
Do not use these substances alternately.
Wipe disinfection
For cleaning and disinfecting the surfaces of the unit or of the equipment cart, Erbe
recommends a wipe disinfection. Use only disinfectant which complies with the rele-
vant national standards.
Instructions for cleaning and disinfection
Mix the disinfectant in the concentration specified by the manufacturer.
Clean surfaces contaminated with blood before using the disinfectant; otherwise it
may be less effective.
Wipe the surfaces. Make sure the surfaces are treated uniformly. Comply with the ac-
tion time of the disinfectant specified by the manufacturer.
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.
143 / 158
16 • Messages
80114-601
03.16
Chapter 16
Messages
A message consists of a title, a message text and a code. The VIO system displays three
different types of messages:
a) Messages that prompt you to inform Technical Service, as the VIO 3 or a VIO module
(e.g. APC 3) cannot be used. These messages are not listed individually in the User
Manual, because the messages only differ in their code. The title and the message text
are all identical: Note: The unit cannot be used. Please contact the service department.
b) Status messages.
c) Messages that prompt you to take action.
Messages of categories b) and c) are found in the following table. The messages are
sorted alphabetically by their code.
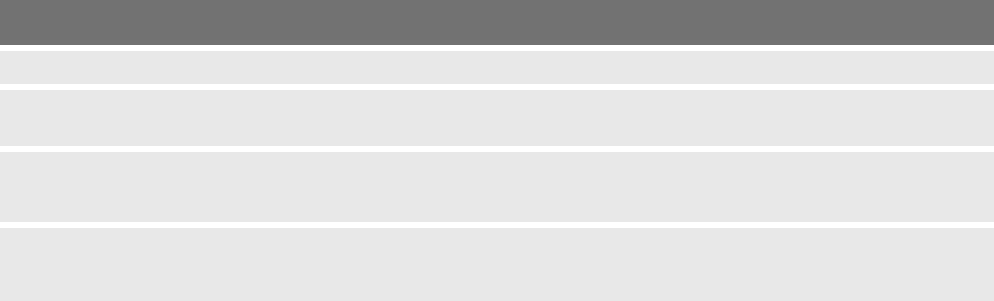
Code Title Message text
G-A-75 High unit temperature The unit has overheated. Activation may only be repeated once the
unit has cooled down.
Please contact the service department.
I-A-30 to
I-A-33
Faulty instrument The instrument is faulty and cannot be used.
I-A-34 Check connection Make sure that the instrument cable is correctly connected to the
instrument and to the unit.
If the connection is correct, then the instrument is faulty and cannot be
used.
I-A-35 Faulty socket or instrument A faulty connector may have been connected at the marked socket.
Remove the connector from this socket.
If the problem persists, contact the service department.
I-A-36 Monopolar socket A faulty connector may have been connected at the marked socket.
Remove the connector from this socket.
If the problem persists, contact the service department.
I-A-37 Bipolar socket The marked socket may be faulty.
Remove the connector from this socket.
If the problem persists, contact the service department.
I-A-40 Button pressed A button was pressed while plugging the instrument.
Insert the instrument without pressing a button.
If the problem persists, replace the instrument.
Otherwise, please contact the service department.
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.

16 • Messages
144 / 158
80114-601
03.16
IES-A-20 IES smoke evacuator The IES smoke evacuator has overheated.
The module cannot be used.
Please contact the service department.
IES-A-21 IES smoke evacuator The IES smoke evacuator has not yet acclimatized.
The module can not yet be used.
IES-A-23 Filter cartridge used up Replace the filter cartridge of the IES evacuator.
IES-A-24 High suction resistance Make sure that the suction hose is free from blockages.
Remove the protective cap or replace the filter.
IES-A-25 Filter cartridge not detected Make sure that the filter cartridge has been correctly inserted.
M-A-1 No tissue effect The hand trigger has been pulled during activation.
Release the hand trigger and repeat activation.
M-A-2 No tissue effect Tissue contact is not sufficient for sealing.
Ensure that there is sufficient tissue between the jaws of the instru-
ment.
Grip the tissue again if required.
M-A-10 No tissue effect Repeat activation and quickly guide the loop towards the tissue.
M-A-11 No tissue effect Ensure that saline solution is used as the irrigation solution.
Activate the instrument in the saline solution.
Check the cable and the connections.
M-A-20 Excessive power An excessive level of power was output.
Guide the instrument quickly to the tissue.
If possible, switch off the QuickStart function.
N-A-48 Return electrode monitoring Which return electrode type have you just connected?
Split return electrode
Non-split return electrode
Information on split and non-split return electrodes
N-A-49 Return electrode monitoring Monitoring cannot detect any contact of a return electrode with the
skin.
If you have connected a return electrode:
Check the cable for damage.
Make sure that the contact strip is correctly positioned in the connect-
ing terminal.
Make sure that the plug of the return electrode cable is correctly
inserted in the unit.
Code Title Message text
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.

145 / 158
16 • Messages
80114-601
03.16
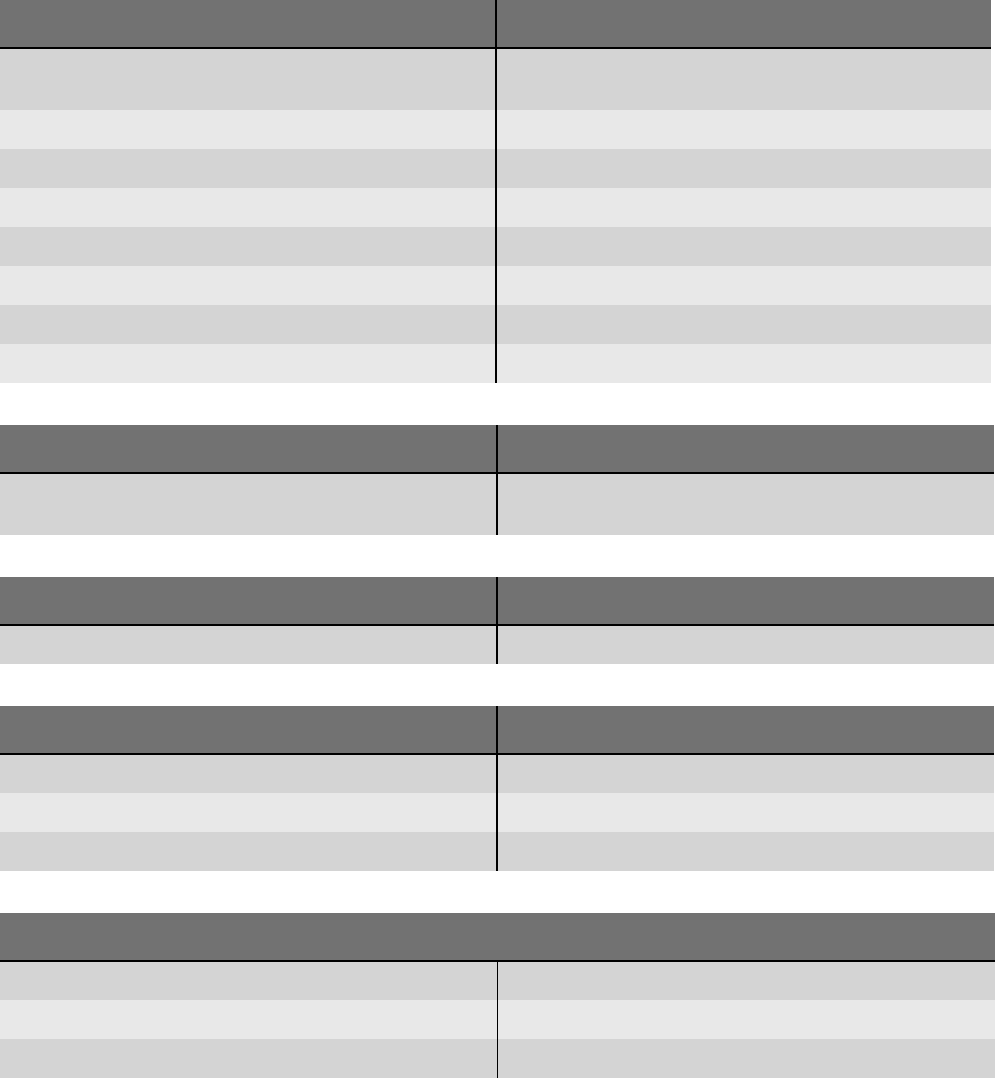
N-A-50 Return electrode monitoring Which return electrode type have you just connected?
Split return electrode
Non-split return electrode
Information on split and non-split return electrodes
N-A-51 Return electrode monitoring Check return electrode!
The skin contact of the return electrode is not sufficient.
Make sure that the complete surface of the return electrode is fully
attached without any creases.
The skin beneath the return electrode must be dry, and free of oil and
hair.
Check the cable for damage.
More information is provided in the User manual.
N-A-52 Return electrode monitoring Check the alignment of the return electrode!
The current is not distributed evenly over the surface of the return
electrode.
Make sure that the long side of the return electrode faces towards the
operating field.
Make sure that the complete surface of the return electrode is fully
attached without any creases.
Check whether the return electrode supports NESSY symmetry moni-
toring.
N-A-53 Return electrode monitoring Check connection!
Very low electrical resistance.
Check the cable for damage.
Make sure that a split return electrode is connected.
N-A-54 Return electrode monitoring The connection between the return electrode and the unit is faulty.
Check the cable for damage.
N-A-55 Return electrode monitoring Which return electrode type have you just connected?
Split return electrode
Non-split return electrode
Information on split and non-split return electrodes
Code Title Message text
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.
16 • Messages
146 / 158
80114-601
03.16
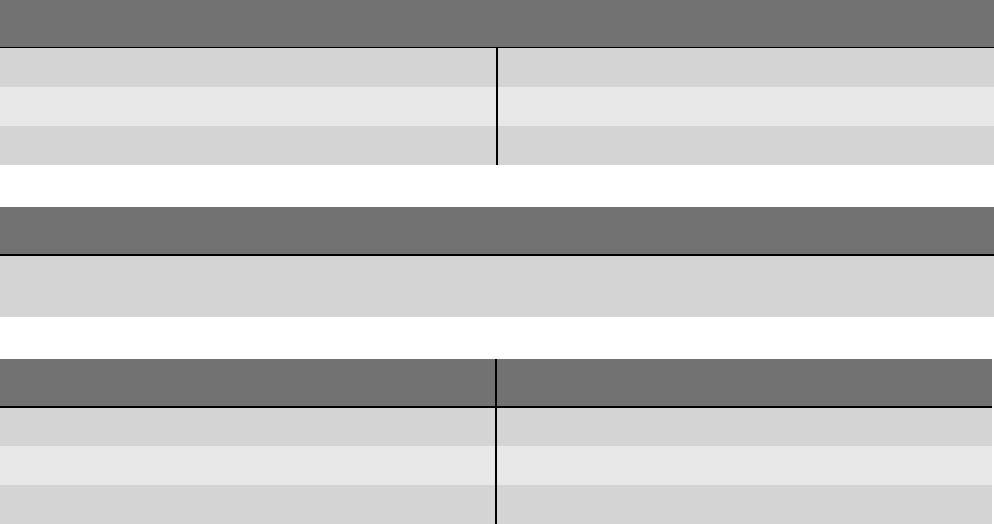
N-A-190 Return electrode monitoring Check the alignment of the return electrode!
The current is not distributed evenly over the surface of the return
electrode.
Make sure that the long side of the return electrode faces towards the
operating field.
Make sure that the complete surface of the return electrode is fully
attached without any creases.
Check whether the return electrode supports NESSY symmetry moni-
toring.
I-A-191
and
I-A-192
Return electrode monitoring A higher temperature is possible under the return electrode!
Activate for as brief a period as possible.
Reduce the effect setting if the situation permits.
S-A-18 High power output High output power was registered over a long period.
Strong heat was applied to internal components.
Activation may only be repeated once the unit has cooled down.
S-A-19 Maximum activation time The maximum activation time has been reached.
You can adjust the duration in the "Protected Settings".
S-A-21 High unit temperature The unit has overheated. Activation may only be repeated once the
unit has cooled down.
S-A-22 Incompatible module An incompatible module was connected to the system.
Disconnect this module from the system or contact the service depart-
ment.
S-A-23 Incompatible module An incompatible module was connected to the system.
Disconnect this module from the system or contact the service depart-
ment.
S-A-24 Contact detected You have assigned AUTO START to an instrument. The unit has already
detected contact with this instrument.
Do not touch any tissue during assignment.
If there is no contact, check the cable and the instrument for damage.
S-A-29 and
S-A-30
Disconnect the footswitch You have connected two identical footswitches. It is only possible to
connect one two pedal footswitch and one one pedal footswitch in
each case.
First disconnect both footswitches from the unit.
S-A-31 Internal module A modification to the internal module configuration has been detected.
Check whether all configured modules in the system have been cor-
rectly detected.
Confirm correct configuration at the service level.
S-A-40 Pedal pressed A pedal on the two-pedal footswitch was pressed during startup.
Do not press any pedal.
If the error persists, replace the footswitch.
Code Title Message text
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.

147 / 158
16 • Messages
80114-601
03.16
S-A-41 Pedal pressed A pedal on the one-pedal footswitch was pressed during startup.
Do not press any pedal.
If the error persists, replace the footswitch.
S-A-129 High unit temperature The unit has heated up considerably.
Activate for as brief a period as possible.
Reduce the effect setting if the situation permits.
If the temperature continues to rise, you may no longer be able to use
the unit.
S-A-130 Incompatible instrument An incompatible instrument was connected to the system.
Disconnect this instrument from the system or contact the service
department.
S-A-149 Two pedals pressed You have pressed both pedals of the two-pedal footswitch at the same
time.
Release the pedals.
If the error persists, replace the footswitch.
S-A-150 Check date / time The date and time may not have been set correctly.
Check the settings in the menu.
S-A-154 Footswitch not assigned You have activated a footswitch that is not assigned to an instrument.
S-A-155 No mode set You have attempted to activate an instrument for which no mode has
been set.
Select a mode and an effect.
S-A-156 End activation Interrupt activation and grip the tissue again.
S-A-157 Instrument not connected You have activated a footswitch that is assigned to an instrument.
However, the instrument is not connected to the unit.
Connect the instrument to a socket.
S-A-166 APC 3 argon plasma module The argon plasma module APC 3 is not ready for operation.
Open the valve of the argon gas bottle.
Ensure that the gas hose and the sensor cable of the pressure regula-
tor are connected at the rear of the APC 3 argon plasma module.
S-A-200 Smoke evacuator detected The IES 2 smoke evacuator was detected by the system and can be
used.
S-A-201 Smoke evacuator disconnected The IES 2 smoke evacuator was disconnected from the system.
U-A-7 Line voltage too low Please contact the service department if the error continues to occur.
U-A-132 Program memory full The maximum number of programs has been reached.
Delete programs that you no longer need in order to save new ones.
U-A-133 Remote control detected VIO 3 is connected with a remote control. Unit settings can be modified
using this remote control.
U-A-134 Remote control disconnected VIO 3 was disconnected from the remote control.
Code Title Message text
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.
16 • Messages
148 / 158
80114-601
03.16
U-A-135 Modification not possible No other mode can be selected for this instrument.
U-A-136 Data transfer active Data is being transferred to the VIO 3. The unit cannot be used at this
time.
U-A-137 Safety check The regular safety check is due.
Please contact the service department.
U-A-138 Assign activation type The newly-connected instrument cannot be activated.
Assign either the footswitch or AUTO START to the instrument.
Code Title Message text
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.
149 / 158
17 • General Technical Data
80114-601
03.16
Chapter 17
General Technical Data
Power connection
Rated supply voltage 100 – 120 VAC (±10%) /
220 – 240 VAC (±10%) /
Rated supply frequency 50 Hz / 60 Hz
Line current (averaged) max. 6.3 A / 2.5 A
Power input in standby mode < 30 watts
Power input with max. HF output 550 watts
Max. pulse power consumption 1600 watts
Terminal for grounding (potential equalization) yes
Power fuses T 6.3 A H / 250 V
Operating mode
Intermittent operation 25% activation time
(e.g. activated for 10 sec. / deactivated for 30 sec.)
WiFi
WiFi yes (deactivated as standard)
Dimensions and weight
Width x height x depth 415 x 215 x 375 mm
Weight 12 kg
Display size 10.4 inch
Ambient conditions for transport and storage of unit
Tem perature -30°C to +70°C
Relative humidity 10% – 90%
Air pressure 540 hPa - 1060 hPa
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.
17 • General Technical Data
150 / 158
80114-601
03.16
Ambient conditions for operation of the unit
Tempera tu re +10°C to +40°C
Relative humidity 15% - 80%, non-condensing
Air pressure 540 hPa - 1060 hPa
Acclimatizing
If the unit has been stored or transported at temperatures below +10 °C or above +40 °C, the unit will require approx. 3 hours
to acclimatize at room temperature.
Standards
Classification according to EC Directive 93/42/EEC II b
Protection class as per EN 60 601-1 I
Type as per EN 60 601-1 CF
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.
151 / 158
18 • Information on electromagnetic compatibility (EMC)
80114-601
03.16
Chapter 18
Information on electromagnetic
compatibility (EMC)
Where EMC is concerned, medical electrical equipment is subject to special safety
measures and must be installed and commissioned according to the EMC instructions
stated herein.
Guidelines for avoiding, recognizing and rectifying unwanted
electromagnetic effects on other equipment or systems, which are the
result of operating the VIO system.
When VIO electrosurgical units are activated, disturbance of other equipment or sys-
tems in the immediate vicinity can occur. This can be recognized as, for example, im-
age artifacts in imaging devices or unusual fluctuations in measured value displays.
Such disturbances from an activated electrosurgical unit can be reduced by placing it
further away and/or carrying out suitable shielding measures on the equipment or
system experiencing disturbance.
When the VIO electrosurgical unit is in the non-activated state, interference with other
equipment in the immediate vicinity does not occur.
NOTICE
Use of non-approved internal cables by Technical Service
This can result in the increased emission of electromagnetic waves or
reduce the immunity of the device.
The unit may fail or not perform properly.
Technical Service may only use the internal cables that are listed
in the service manual for the device.
NOTICE
Stacked devices
If you stack the device next to other equipment or with other equip-
ment, the devices can affect each other.
The unit may fail or not perform properly.
The device may only be stacked next to or with VIO series units.
If it is necessary to operate the device near other equipment or
stacked together with other equipment, check whether the devic-
es are affecting each other: Are the devices behaving unusually?
Do errors occur?
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.
18 • Information on electromagnetic compatibility (EMC)
152 / 158
80114-601
03.16
Guidance and manufacturer's declaration - electromagnetic emissions
The equipment is intended for use in the electromagnetic environment specified below. The customer or the user of the
equipment should ensure that it is used in such an environment.
Emissions test Compliance Electromagnetic environment - guidance
HF emissions CISPR 11 Group 1 The equipment or system uses HF energy only for its
internal function. Therefore its HF emissions are very
low and are not likely to cause any interference in
nearby electronic equipment.
HF emissions CISPR 11 Class A The unit is suited for use in environments other than
domestic areas and in ones directly connected to a
public power supply system that also supplies build-
ings being used for domestic purposes.
Harmonic emissions IEC 61000-3-2 Class A
Voltage fluctuations/flicker emissions IEC
61000-3-3
Complies
Guidance and manufacturer's declaration - electromagnetic immunity
The equipment is intended for use in the electromagnetic environment specified below. The customer or the user of the
equipment should ensure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment
- guidance
Electrostatic discharge
(ESD) IEC 61000-4-2
±6 kV contact
±8 kV air
±6 kV contact
±8 kV air
Floors should be wood, con-
crete or ceramic tile. If floors
are covered with non-conduc-
tive synthetic material, the rela-
tive humidity should be at least
30%.
Electrical fast tran-
sient/burst IEC 61000-
4-4
±2 kV for power supply lines
±1 kV for input/output lines
±2 kV for power supply lines
±1 kV for input/output lines
Mains power quality should be
that of a typical commercial or
hospital environment.
Surge IEC 61000-4-5 ±1 kV differential mode
±2 kV common mode
±1 kV differential mode
±2 kV common mode
Mains power quality should be
that of a typical commercial or
hospital environment.
Voltage dips, short
interruptions and volt-
age variations on
power supply input
lines IEC 61000-4-11
<5% U T (>95% dip in U T ) for
0.5 cycle
40% U T (60% dip in U T ) for
5 cycles
70% U T (30% dip in U T ) for
25 cycles
<5% U T (>95% dip in U T ) for
5 s
<5% U T (>95% dip in U T )
for 0.5 cycle
40% U T (60% dip in U T ) for
5 cycles
70% U T (30% dip in U T ) for
25 cycles
<5% U T (>95% dip in U T )
for 5 s
Mains power quality should be
that of a typical commercial or
hospital environment.
If the user of the equipment
requires continued operation
during power mains interrup-
tions, it is recommended that
the equipment be powered
from an uninterruptible power
supply or a battery.
Power frequency (50/
60 Hz) magnetic field
IEC 61000-4-8
3 A/m 3 A/m Power frequency magnetic
fields should be at levels char-
acteristic of a typical location in
a typical commercial or hospital
environment.
Note: UT is the a.c. mains voltage prior to application of the test level.
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.

153 / 158
18 • Information on electromagnetic compatibility (EMC)
80114-601
03.16
Guidance and manufacturer's declaration - electromagnetic immunity
The equipment is intended for use in the electromagnetic environment specified below. The customer or the user of the
equipment should ensure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment -
guidance
Portable and mobile HF commu-
nications equipment should be
used no closer to any part of the
equipment, including cables, than
the recommended separation
distance. The separation dis-
tance is calculated from various
equations depending on the fre-
quency of the portable and
mobile HF communications
equipment:
Recommended separation dis-
tance
Conducted HF IEC 61000-
4-6
3 Vrms
150 kHz to 80 MHz
3 Vrms Equation 1) d=1.2 P1/2
Radiated HF IEC 61000-4-
3
3 V/m
80 MHz to 800 MHz
3 V/m Equation 2) d=1.2 P1/2
3 V/m
800 MHz to 2.5 GHz
3 V/m Equation 3) d=2.3 P1/2
P is the maximum output power
rating of the transmitter in watts
(W) according to the transmitter
manufacturer. d is the recom-
mended separation distance in
meters (m).
Field strengths from fixed trans-
mitters, as determined by an
electromagnetic site surveya)
should be less than the compli-
ance level in each frequency
rangeb).
Interference may occur in the
vicinity of equipment marked
with the following symbol:
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.
18 • Information on electromagnetic compatibility (EMC)
154 / 158
80114-601
03.16
Note 1: At 80 MHz equation 2) applies. At 800 MHz equation 3) applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection
from structures, objects and people.
a) Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile
radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To
assess the electromagnetic environment due to fixed HF transmitters, an electromagnetic site survey should be considered. If
the measured field strength in the location in which the equipment is used exceeds the applicable compliance level above,
the equipment should be observed to verify normal operation. If abnormal performance is observed, additional measures
may be necessary, such as reorienting or relocating the equipment.
b) Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Recommended separation distances between portable and mobile HF communications equipment and the
equipment
The equipment is intended for use in an electromagnetic environment in which radiated HF disturbances are controlled. The
customer or the user of the equipment can help prevent electromagnetic interference. This can be achieved by maintaining
the minimum distance recommended below between the communications equipment (transmitters) and the equipment. The
minimum distance depends on the maximum output power and the frequency of the communications equipment.
Rated maximum output power
of transmitter (W)
Separation distance according to frequency of transmitter (m)
150 kHz to 80 MHz
d=1.2 P1/2 80 kHz to 800 MHz
d=1.2 P1/2 800 MHz to 2.5 GHz
d=2.3 P1/2
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
11.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance can be deter-
mined using the equation applicable to the frequency of the transmitter. P is the maximum output power rating of the trans-
mitter in watts (W) according to the transmitter manufacturer.
Note 1: An additional factor of 10/3 is used in calculating the recommended separation distance for transmitters in the fre-
quency bands between 80 MHz and 2.5 GHz to decrease the likelihood that mobile/portable communications equipment
could cause interference if it is inadvertently brought into patient areas.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection
from structures, objects and people.
Guidance and manufacturer's declaration - electromagnetic immunity
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.
155 / 158
19 • WiFi explanations
80114-601
03.16
Chapter 19
WiFi explanations
Explanation on compliance with FCC Rules
Applies for the USA and all countries oriented toward FCC certification.
This device is compliant with the RSS radio standard from Industry Canada for license
exempt devices, as well as Part 15 FCC Rules. Operation is subject to the following two
conditions: (1) The device must not cause any interference and (2) the device must ac-
cept all interference, even interference that could cause undesired operation. Changes
or modifications not expressly approved by the parties responsible for compliance
could void the user's authority to operate the equipment.
FCC:2AGEM-VIO3
Explanation on compliance with IC Rules
Applies for Canada.
This device is compliant with the CNR regulations from Industry Canada applicable for
license exempt devices. Operation is subject to the following two conditions: (1) The
device must not cause any interference and (2) the device must accept all radio fre-
quency interference, even interference that could impair operation.
Le présent appareil est conforme aux CNR d'Industrie Canada applicables aux
appareils radio exempts de licence. L'exploitation est autorisée aux deux conditions
suivantes : (1) l'appareil ne doit pas produire de brouillage, et (2) l'appareil doit
accepter tout brouillage radioélectrique subi, même si le brouillage est susceptible
d'en compromettre le fonctionnement.
IC:20687-VIO3
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.
This equipment has been tested and found to comply with the limits for a Class A
digital device, pursuant to part 15 of the FCC Rules. These limits are designed to
provide reasonable protection against harmful interference when the equipment is
operated in a commercial environment. This equipment generates, uses, and can
radiate radio frequency energy and, if not installed and used in accordance with
the instruction manual, may cause harmful interference to radio communications.
Operation of this equipment in a residential area is likely to cause harmful
interference in which case the user will be required to correct the interference at
his own expense.
19 • WiFi explanations
156 / 158
80114-601
03.16
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.

157 / 158
20 • Maintenance, Customer Service, Warranty, Disposal
80114-601
03.16
Chapter 20
Maintenance, Customer Service, Warranty,
Disposal
Maintenance
Modifications and repairs Modifications and repairs must not impair the safety of the equipment or equipment
cart and accessories for the patient, user and the environment. This condition is met
when changes to the structural and functional characteristics are not detrimental to
safety.
Authorized persons Modifications and repairs may only be undertaken by Erbe or by persons expressly au-
thorized by Erbe. Erbe accepts no liability if modifications and repairs to the unit or
accessories are made by unauthorized persons. This will also invalidate the warranty.
Technical safety checks The technical safety checks determine whether the safety and operational readiness
of the unit or the equipment cart and accessories conform to a defined technical re-
quired status. Technical safety checks must be performed at least once a year.
What technical safety checks must
be performed?
For this device the following technical safety checks have been stipulated:
•Checking of labels and User Manual
•Visual inspection of unit and accessories for damage
•Testing the grounded conductor as per EN 62353
•Leakage current testing as per EN 62353
•Measurement of DC resistance
•Functional testing of all the unit's operating and control elements
•Testing footswitch and fingerswitch activation
•Testing instrument and connector detection
•Testing the automatic start mode
•Measurement of the HF peak voltage for sinusoidal and modulated modes
•Measurement of the output power in the CUT and COAG operating modes
•Testing the monitoring circuits (monitoring equipment)
The results of the safety checks must be documented.
If during the safety checks any defects are found which might endanger patients, staff
or third parties, the device may not be operated until the defects have been remedied
by competent service technicians.
Customer service
If you are interested in a maintenance contract, please contact Erbe Elektromedizin in
Germany, or your local contact in other countries. This may be an Erbe subsidiary, an
Erbe representative or a distributor.
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.

20 • Maintenance, Customer Service, Warranty, Disposal
158 / 158
80114-601
03.16
Warranty
The General Terms and Conditions or the conditions of the purchase contract apply.
Disposal
Your product bears a crossed-out garbage can icon (see image). Meaning: In all EU
countries this product must be disposed of separately in accordance with the national
laws implementing EU Directive 2002/96/EC of January 27, 2003, WEEE.
In non-EU countries the local regulations must be observed.
If you have any questions about disposal of the product, please contact Erbe Elektro-
medizin or your local distributor.
Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.Dok.-Nr: D110127-EN, Ver.: 000, ÄM-Nr: 16446, Gültig ab: 10.05.16, Gedruckt: MZECEVIC/02.06.16, Ausdruck nicht maßstäblich und kein Original.