St Jude Medical SJMRF Rf Implantable Medical Device User Manual
St. Jude Medical Rf Implantable Medical Device
Contents
User Manual

Current™/Current RF
DR Model 2107–36/30
RF DR Model 2207–36/30
VR Model 1107–36/30
RF VR Model 1207–36/30
Implantable Cardioverter-Defibrillator
Promote™/Promote RF
Model 3107-36/30
RF Model 3207-36/30
Cardiac Resynchronization Device,
Implantable Cardioverter-Defibrillator
User’s Manual
DRAFT
CAUTION
Federal (USA) law restricts this device to sale by or
on the order of a physician.
© 2007 St. Jude Medical Cardiac Rhythm Management Division.
All Rights Reserved.
Unless otherwise noted, ® or ™ indicates that the name is a trademark of,
or licensed to, St. Jude Medical, Inc. or its subsidiaries.
DRAFT
Current™ and Promote™ Devices User’s Manual 1
Device Description
This manual describes the following St. Jude Medical® pulse generators:
The pulse generator, along with compatible, commercially available leads, con-
stitutes the implantable portion of the ICD and CRT-D systems. The lead sys-
tems are implanted using either transvenous or transthoracic techniques. The
St. Jude Medical Merlin™ Patient Care System (PCS) with software version 6.0
(or greater), a Merlin Antenna (for devices with RF commnication), and a telem-
etry wand constitute the external portion of the ICD and CRT-D systems.
Indications and Usage
The Current™ and Promote™ pulse generators are intended to provide ventric-
ular antitachycardia pacing and ventricular defibrillation for automated treat-
ment of life-threatening ventricular arrhythmias.
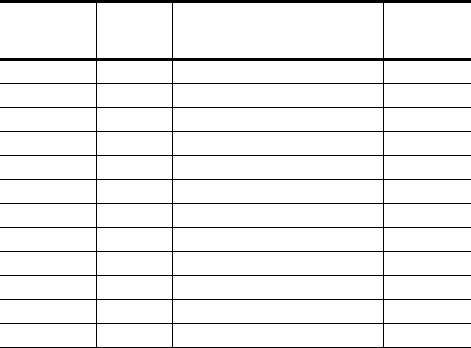
Name Model # Description Delivered
Energy
(approx.)
Current DR 2107-36 Dual-chamber ICD 36 J
Current DR 2107-30 Dual-chamber ICD 30 J
Current VR 1107-36 Single-chamber ICD 36 J
Current VR 1107-30 Single-chamber ICD 30 J
Current RF DR 2207-36 Dual-chamber ICD with RF telemetry 36 J
Current RF DR 2207-30 Dual-chamber ICD with RF telemetry 30 J
Current RF VR 1207-36 Single-chamber ICD with RF telemetry 36 J
Current RF VR 1207-30 Single-chamber ICD with RF telemetry 30 J
Promote 3107-36 CRT-D 36 J
Promote 3107-30 CRT-D 30 J
Promote RF 3207-36 CRT-D with RF telemetry 36 J
Promote RF 3207-30 CRT-D with RF telemetry 30 J
Table 1. Current™ and Promote™ pulse-generator descriptions
DRAFT
2 Contraindications
AF suppression pacing is indicated for suppression of paroxysmal or persistent
atrial fibrillation in patients with the above ICD indication and sinus node dys-
function.
In patients indicated for an ICD, the Promote pulse generators are also
intended:
• to provide a reduction of the symptoms of moderate to severe heart failure
(NYHA Functional Class III or IV) in those patients who remain symptomatic
despite stable, optimal medical therapy (as defined in the clinical trials sec-
tion included in the Merlin PCS on-screen help) and have a left ventricular
ejection fraction less than or equal to 35% and a prolonged QRS duration
• to maintain synchrony of the left and right ventricles in patients who have
undergone an AV nodal ablation for chronic (permanent) atrial fibrillation
and have NYHA Class II or III heart failure
Contraindications
Contraindications for use of the pulse generator system include ventricular
tachyarrhythmias resulting from transient or correctable factors such as drug
toxicity, electrolyte imbalance, or acute myocardial infarction.
Warnings and Precautions
Resuscitation Availability. Do not perform device testing unless an external
defibrillator and medical personnel skilled in cardiopulmonary resuscitation
(CPR) are readily available.
Lead system. Do not use another manufacturer’s lead system without demon-
strated compatibility as undersensing cardiac activity and failure to deliver nec-
essary therapy may result.
Avoiding shock during handling. Disable tachyarrhythmia therapy (Enable/
Disable Tachy Therapy) or program tachyarrhythmia therapies Off during surgi-
cal implant and explant or post-mortem procedures as well as when discon-
necting leads as the device can deliver a serious shock if you touch the
defibrillation terminals while the device is charged.
Additional pacemaker implanted. These devices provide bradycardia pacing.
If another pacemaker is used, it should have a bipolar pacing reset mode and
be programmed for bipolar pacing to minimize the possibility of the output
pulses being detected by the device.
Modifying the device. This device has been tested for compliance to FCC reg-
ulations. Changes or modifications of any kind not expressly approved by
St. Jude Medical Inc. could void the user’s authority to operate this device.
DRAFT
Current™ and Promote™ Devices User’s Manual 3
Suboptimal radio frequency (RF) communication. The Merlin PCS indicates
the quality of the RF communication by the telemetry strength indicator LEDs
on both the Merlin PCS and the Merlin Antenna. Below is a list of potential
causes to suboptimal radio communication:
Sterilization, Storage and Handling
Resterilization. Do not resterilize and re-implant explanted pulse generators.
Use before date. Do not implant the device after the “use before” date
because the battery may have reduced longevity.
If package is damaged. Do not use the device or accessories if the packaging
is wet, punctured, opened or damaged because the integrity of the sterile
packaging may be compromised. Return the device to St. Jude Medical.
Device storage. Store the device in a clean area, away from magnets, kits con-
taining magnets, and sources of electromagnetic interference (See ”Environ-
mental and Medical Therapy Hazards” on page 5) to avoid device damage.
Store the device between 10° and 45°C because temperatures outside this
range may damage the device.
Temperature Equilibration. After cold storage, allow the device to reach
room temperature before charging the capacitors, programming, or implanting
the device because cold temperature may affect initial device function.
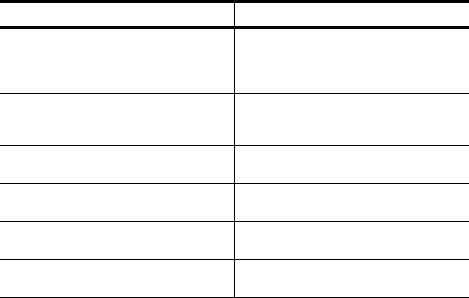
Possible Causes Solutions
The Merlin Antenna orientation/location is
suboptimal.
Move or reorient the Merlin Antenna
slightly. Make sure that the front of the
Merlin Antenna faces the implantable
device.
People or objects interfere with the
communication between the Merlin
Antenna and the device.
Make sure that the space between the
Merlin Antenna and the device is free from
interfering objects/people.
The Merlin Antenna is too far away from
the device.
Move the Merlin Antenna closer to the
device.
Someone is holding the Merlin Antenna. Place the Merlin Antenna on a flat surface.
Do not hold the Merlin Antenna.
Other products in the vicinity are causing
electromagnetic interference (EMI).
Power off or remove equipment that could
cause EMI.
The Merlin Antenna cable is wound
around the Merlin Antenna.
Make sure the Merlin Antenna cable is not
wound around the Merlin Antenna.
Table 2. Possible causes and solutions for suboptimal RF communication
DRAFT

4 Warnings and Precautions
Follow-up Testing
Ensure that an external defibrillator and medical personnel skilled in cardiopul-
monary resuscitation (CPR) are present during post-implant device testing
should the patient require external rescue.
Be aware that the changes in the patient’s condition, drug regimen, and other
factors may change the defibrillation threshold (DFT), which may result in non-
conversion of the arrhythmia. Successful conversion of ventricular fibrillation or
ventricular tachycardia during arrhythmia conversion testing is no assurance
that conversion will occur post-operatively.
Implantation and Device Programming
Do not position a magnet over the device as that suspends detection and treat-
ment (unless the device has been programmed to ignore the magnet).
Replace the device when the battery voltage reaches 2.45 V.
Implant the pulse generator no deeper than 5 cm to ensure reliable data trans-
mission. For patient comfort, do not implant the pulse generator within
1.25 cm of bone unless you cannot avoid it.
Program the device parameters as specified in the Merlin PCS on-screen help.
The results of the DAVID Study demonstrated that, for patients with standard
indications for ICD therapy, no indication for cardiac pacing and an EF < 40%,
dual-chamber pacing offers no clinical advantage over backup VVI pacing and
may be associated with worsening heart failure.1 When programming the
device to dual-chamber pacing modes, give particular attention to setting the
pacing parameters (such as the A-V delay) to promote intrinsic conduction and
minimize the amount of ventricular pacing.
Pulse Generator Explant and Disposal
Interrogate the device and turn all therapies off before explanting, cleaning or
shipping the device to prevent unwanted shocks.
Return all explanted pulse generators and leads to St. Jude Medical.
Never incinerate the device because of the potential for explosion. Explant the
device before cremation.
1. Wilkoff BL, Cook JR, Epstein AE, Greene L, Hallstrom AP, Hsia H, Kutalek SP, Sharma A.
Dual-Chamber Pacing or Ventricular Backup Pacing in Patients With an Implantable
Defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA.
December 25, 2002; Vol 288, No. 24:3115-3123.
DRAFT
Current™ and Promote™ Devices User’s Manual 5
Environmental and Medical Therapy Hazards
Instruct patients to avoid devices which generate a strong electric or magnetic
interference (EMI). EMI could cause device malfunction or damage, resulting in
non-detection or delivery of unneeded therapy. Moving away from the source
or turning it off will usually allow the pulse generator to return to its normal
mode of operation.
Hospital and Medical Environments
Electrosurgical cautery. Electrosurgical cautery could induce ventricular
arrhythmias and/or fibrillation, or may cause device malfunction or damage. If
electrocautery is necessary, keep the current path and groundplate as far away
from the pulse generator and leads as possible.
External defibrillation. External defibrillation may damage the pulse genera-
tor or may result in temporary and/or permanent myocardial damage at the
electrode-tissue interface as well as temporarily or permanently elevated pacing
capture thresholds. Minimize current flowing through the pulse generator and
lead system by following these precautions when using external defibrillation
on a patient with a pulse generator:
• Position defibrillation paddles as far from the pulse generator as possible
(minimum of 13 cm)
• Use the lowest clinically appropriate energy output
• Confirm pulse generator function following any external defibrillation
High radiation sources. Do not direct high radiation sources such as cobalt
60 or gamma radiation at the pulse generator. If a patient requires radiation
therapy in the vicinity of the pulse generator, place lead shielding over the
device to prevent radiation damage and confirm its function after treatment.
Lithotripsy. Lithotripsy may permanently damage the pulse generator. Avoid it
unless the therapy site is not near the pulse generator and leads.
Diathermy. Avoid diathermy, even if the device is programmed off, as it may
damage tissue around the implanted electrodes or may permanently damage
the pulse generator.
Magnetic resonance imaging (MRI). MRI for patients with implantable pulse
generators has been contraindicated by MRI manufacturers. Clinicians should
carefully weigh the decision to use MRI with ICD/CRT-D patients. MRI may
cause device malfunction or injury to the patient.
Ultrasound therapy. Diagnostic and therapeutic ultrasound treatment is not
known to affect the function of the pulse generator.
Transcutaneous Electrical Nerve Stimulation (TENS). TENS may interfere
with device function. To reduce interference, place the TENS electrodes close to
one another and as far from the device/lead system as possible. Monitor cardiac
activity during TENS use.
DRAFT

6 Warnings and Precautions
RF Operating Frequencies. Nearby equipment emitting strong magnetic
fields can interfere with RF communication, even if the other equipment com-
plies with CISPR emission requirements. The operating characteristics are as fol-
lows:
MICS band: 402-405 MHz. The effective radiated power is below the limits as
specified in:
• Europe: EN ETSI 301 839-2
• USA: FCC 47 CFR Part 95; 95.601-95.673 Subpart E, 95.1201-95.1219.
• FCC ID: RIASJMRF.
Radiofrequency ablation. RF ablation in a patient with a pulse generator may
cause device malfunction or damage.
Minimize RF ablation risks by:
• Programming all tachyarrhythmia therapies off
• Avoiding direct contact between the ablation catheter and the implanted
lead or pulse generator
WARNING
This transmitter is authorized by rule under
the Medical Implant Communications Service
(part 95 of the FCC Rules) and must not cause
harmful interference to stations operating in
the 400.150 - 406.000 MHz band in the Meteo-
rological Aids (that is, transmitters and
receivers used to communicate weather
data), the Meteorological Satellite, or the
Earth Exploration Satellite Services and must
accept interference that may be caused by
such aids, including interference that may
cause undesired operation. This transmitter
shall be used only in accordance with the FCC
Rules governing the Medical Implant Commu-
nications Service. Analog and digital voice
communications are prohibited. Although
this transmitter has been approved by the
Federal Communications Commission, there
is no guarantee that it will not receive inter-
ference or that any particular transmission
from this transmitter will be free from inter-
ference.
DRAFT
Current™ and Promote™ Devices User’s Manual 7
• Positioning the groundplate so that the current pathway does not pass
near the pulse generator system, i.e., place the groundplate under the
patient’s buttocks or legs
• Having external defibrillation equipment available.
Home and Occupational Environments
High-voltage power transmission lines. High-voltage power transmission
lines may generate enough EMI to interfere with pulse generator operation if
approached too closely.
Communication equipment. Communication equipment such as microwave
transmitters or high-power amateur transmitters may generate enough EMI to
interfere with pulse generator operation if approached too closely.
Home appliances. Home appliances in good working order and properly
grounded do not usually produce enough EMI to interfere with pulse generator
operation. There are reports of pulse generator disturbances caused by electric
hand tools or electric razors used directly over the pulse generator implant site.
Industrial equipment. A variety of industrial equipment produce EMI of suffi-
cient field strength and modulation characteristics to interfere with proper
operation of the pulse generator. These include, but are not limited to: arc
welders; induction furnaces; very large or defective electric motors; and internal
combustion engines with poorly shielded ignition systems.
Electronic Article Surveillance (EAS)
Advise patients that the Electronic Article Surveillance/Anti-theft (EAS) systems
such as those at the point of sale and entrances/exits of stores, libraries, banks,
etc., emit signals that may interact with the device. It is very unlikely that these
systems will interact with their device significantly. However, to minimize the
possibility of interaction, advise patients to simply walk through these areas at a
normal pace and avoid lingering near or leaning on these systems.
Metal Detectors
Advise patients that metal detector security systems such as those found in air-
ports and government buildings emit signals that may interact with ICDs and
CRT-Ds. It is very unlikely that these systems will interact with their device sig-
nificantly. To minimize the possibility of interaction, advise patients to simply
walk through these areas at a normal pace and avoid lingering. Even so, the
ICD and CRT-D systems contain metal that may set off the airport security sys-
tem alarm. If the alarm does sound, the patient should present security person-
nel with their patient identification card. If security personnel perform a search
with a handheld wand, the patient should ask that they perform the search
DRAFT
8 Warnings and Precautions
quickly, stressing that they should avoid holding the wand over the device for a
prolonged period.
Cellular Phones
The pulse generator has been tested for compatibility with handheld wireless
transmitters in accordance with the requirements of AAMI PC69. This testing
covered the operating frequencies (450 MHz - 3 GHz) and pulsed modulation
techniques of all of the digital cellular phone technologies in worldwide use
today. Based on the results of this testing, the pulse generator should not be
affected by the normal operation of cellular phones.
Potential Adverse Events
Possible adverse events (in alphabetical order) associated with the system,
include, but are not limited to the following:
• Acceleration of arrhythmias (caused by device)
• Air embolism
• Allergic reaction
• Bleeding
• Cardiac tamponade
• Chronic nerve damage
• Death
•Erosion
• Exacerbation of heart failure
• Excessive fibrotic tissue growth
• Extracardiac stimulation (phrenic nerve, diaphragm, chest wall)
• Extrusion
• Fluid accumulation
• Formation of hematomas or cysts
• Inappropriate shocks
•Infection
• Keloid formation
• Lead abrasion and discontinuity
• Lead migration/dislodgment
• Myocardial damage
• Pneumothorax
• Shunting current or insulating myocardium during defibrillation with inter-
nal or external paddles
• Potential mortality due to inability to defibrillate or pace
• Thromboemboli
DRAFT
Current™ and Promote™ Devices User’s Manual 9
• Venous occlusion
• Venous or cardiac perforation.
Patients susceptible to frequent shocks despite antiarrhythmic medical manage-
ment may develop psychological intolerance to an ICD or CRT-D system that
may include the following:
• Dependency
• Depression
• Fear of premature battery depletion
• Fear of shocking while conscious
• Fear of losing shock capability
• Imagined shocking (phantom shock).
Clinician Use Information
Physician Training
Physicians should be familiar with sterile pulse generator implant procedure
and with follow-up evaluation and management of patients with an ICD or
CRT-D (or should refer the patient to such a physician).
Directions for Use
Pulse generator operating characteristics should be verified at the time of
implantation and recorded in the patient file. Complete the Patient Registration
Form and return it to St. Jude Medical as it provides necessary information for
warranty purposes and patient tracking.
Copies of this user’s manual can be obtained by contacting your St. Jude Medi-
cal representative.
Maintaining Device Effectiveness
Device storage
FOR SINGLE USE ONLY. Do not resterilize and re-implant explanted pulse gen-
erators.
St. Jude Medical has sterilized the pulse generator with ethylene oxide prior to
shipment. Contact St. Jude Medical if resterilization is necessary.
Do not implant the device when:
• It has been dropped on a hard surface because this could have damaged
pulse generator components.
DRAFT

10 Clinician Use Information
• The sterility indicator within the inner package is purple, because it might
not have been sterilized.
• Its storage package has been pierced or altered, because this could have
rendered it non-sterile.
• It has been stored or transported outside the environmental temperature
limits.
Storage limits: 10° to 45°C.
Transportation limits: -20° to 60°C.
An electrical reset condition may occur at temperatures below -20°C.
After cold storage, allow the device to reach room temperature before
charging the capacitors, programming, or implanting the device because
cold temperature may affect initial device function.
• Its “use before” date has expired, because this can adversely affect pulse
generator longevity or device sterility.
Do not resterilize the pulse generator using an autoclave, gamma-irradiation,
organic cleaning agents (e.g., alcohol, acetone, etc.), or ultrasonic cleaners.
Sterilization Instructions
Contact St. Jude Medical if resterilization is necessary.
Radiopaque Identification
Each pulse generator has an x-ray absorptive marker for non-invasive identifica-
tion. The two-letter model code is visible on a radiograph.
Figure 3. Current™ and Promote™ devices x-ray marker
Figure 4. Current RF and Promote RF devices x-ray marker
DRAFT
Current™ and Promote™ Devices User’s Manual 11
Package Contents
The pulse generator is supplied in a sterile tray for introduction into the operat-
ing field. The tray contains:
• One pulse generator (with all tachyarrhythmia therapies off) with pre-
installed setscrews
• Torque driver.
The outer box contains:
• Literature.
Technical Service
St. Jude Medical Cardiac Rhythm Management Division maintains 24-hour
phone lines for technical questions and product support:
• by phone for bradycardia or tachycardia devices
- 1 818 364 1506 or
- 1 800 722 3774 (toll-free within North America)
• by fax for bradycardia devices
- 1 818 362 7182 or
- 1 800 756 7223 (toll-free within North America)
• by fax for tachycardia devices
- 1 408 522 6755 or
- 1 866 739 0040 (toll-free within North America).
For additional assistance, call your local St. Jude Medical representative.
Additional Information
For additional information on this device, see the Merlin PCS on-screen help.
DRAFT
12 Additional Information
DRAFT
DRAFT

May 2007
Art 60012839/001
Cardiac Rhythm Management Division
15900 Valley View Court
Sylmar, CA 91342 USA
+1 818 362 6822
701 E. Evelyn Avenue
Sunnyvale, CA 94086 USA
+1 408 738 4883
Veddestavägen 19
SE-175 84 Järfälla
Sweden
+46 8 474 4000
DRAFT