Roche Diabetes Care 876 Hand Held Blood Glucose Meter User Manual manual pt 4
Roche Diagnostics Operations, Inc. Hand Held Blood Glucose Meter manual pt 4
Contents
- 1. manual pt 1
- 2. manual pt 2
- 3. manual pt 3
- 4. manual pt 4
manual pt 4
Data Transfer and PC Reports
71
8
Overview
You have 2options to display and analyze blood glucose results on a PC.
1. Data Transfer – this option transfers the data to special software for diabetes management in a PC.
2. PC Reports – the meter generates data reports that open in an Internet browser and can be printed.
The option you select remains as the default until you change it.
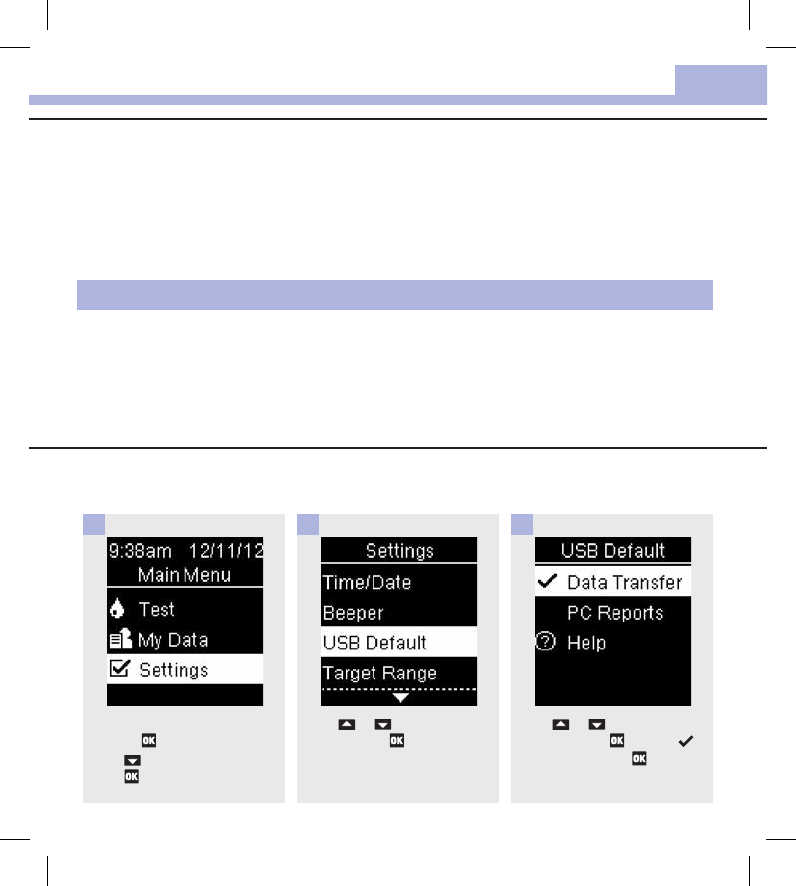
Turn the meter on by briefly
pressing . From Main Menu,
press to highlight Settings.
Press .
1
Press or to highlight USB
Default. Press .
2
Press or to highlight Data
Transfer. Press to move
to the option. Press to set the
option and return to Settings.
3
Chapter 8: Data Transfer and PC Reports
NOTE
• You cannot perform a blood glucose test while the meter is connected to a computer with a USB cable.
If you connect the meter to a PC when a test is in progress, the test is cancelled.
• The meter has a port on the side for the small end of the USB cable. The large end of the USB cable is
inserted into the USB port of a PC.
Set Data Transfer as the Default (Main Menu > Settings > USB Default > Data
Transfer)
52195_AvivaConnect_FDA.indb 71 5/1/14 7:23 AM
Data Transfer and PC Reports
72
8
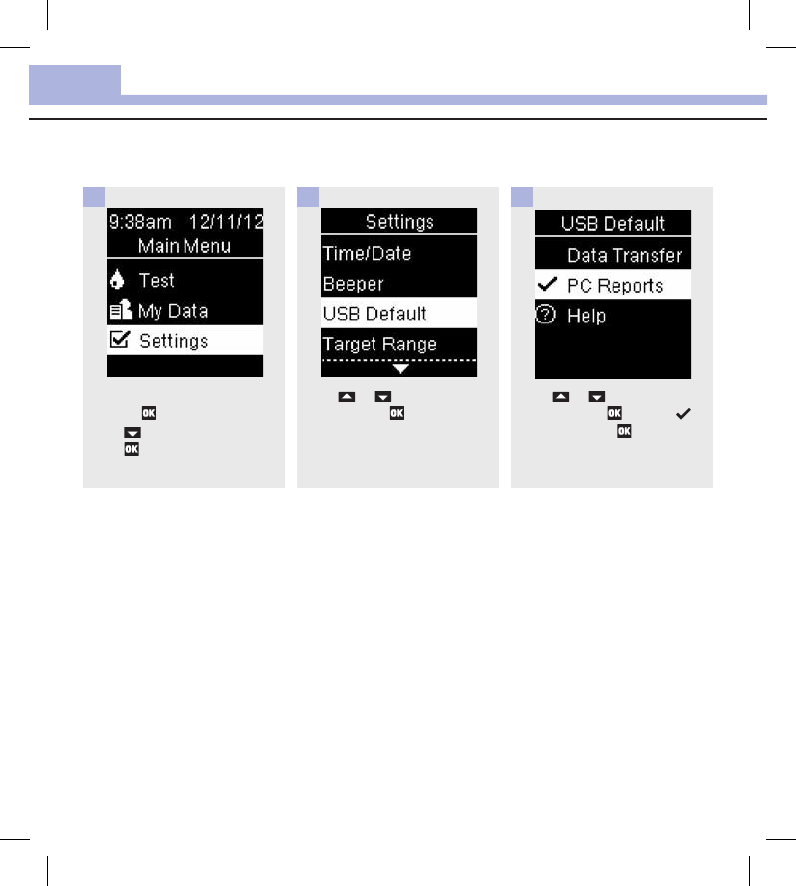
Set PC Reports as the Default (Main Menu > Settings > USB Default > PC Reports)
Turn the meter on by briefly
pressing . From Main Menu,
press to highlight Settings.
Press .
1
Press or to highlight USB
Default. Press .
2
Press or to highlight PC
Reports. Press to move to
the option. Press to set the
option and return to Settings.
3
52195_AvivaConnect_FDA.indb 72 5/1/14 7:23 AM
Data Transfer and PC Reports
73
8
View the Data in Compatible Diabetes Management Software
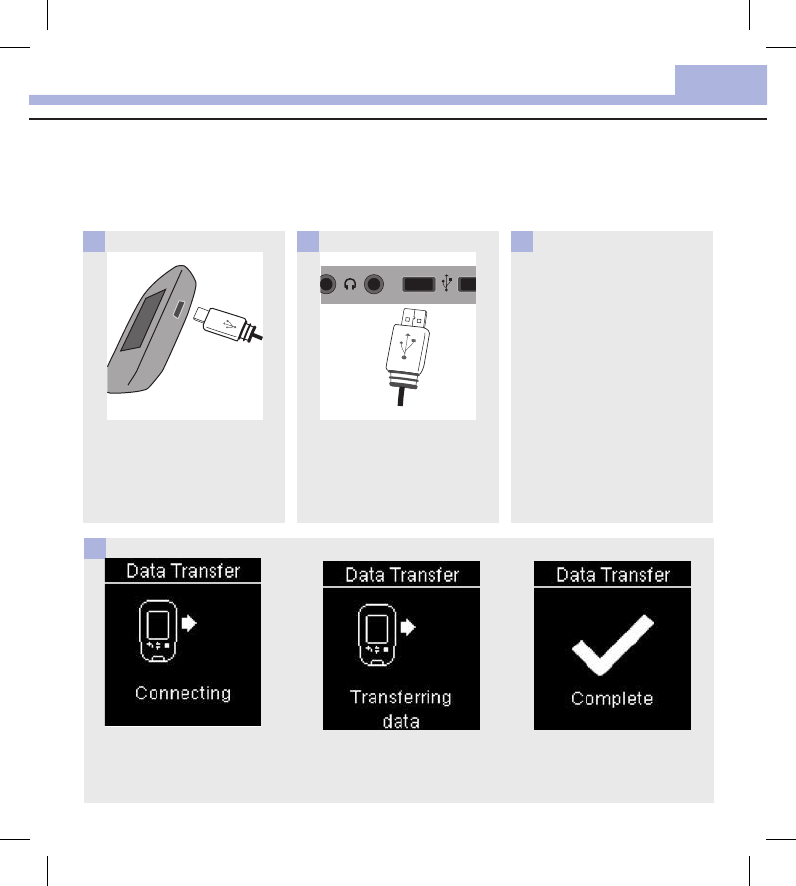
These steps assume that the USB default option is set to Data Transfer.
The meter can be o or on.
Plug the small end of the USB
cable into the meter.
1
Plug the large end of the USB
cable into a USB port on a PC.
If the meter is o, it turns on.
2
4
Start the software for data
analysis and initiate a data
transfer.
3
The meter transfers the data
to the software.
52195_AvivaConnect_FDA.indb 73 5/1/14 7:23 AM
Data Transfer and PC Reports
74
8
NOTE
If you would like to view the data in PC Reports instead, follow these steps:
1. Unplug the USB cable. Connection Lost appears.
2. Press to return to Main Menu.
3. Select My Data>PC Reports.
4. Reconnect the USB cable.
5. PC Reports appears.
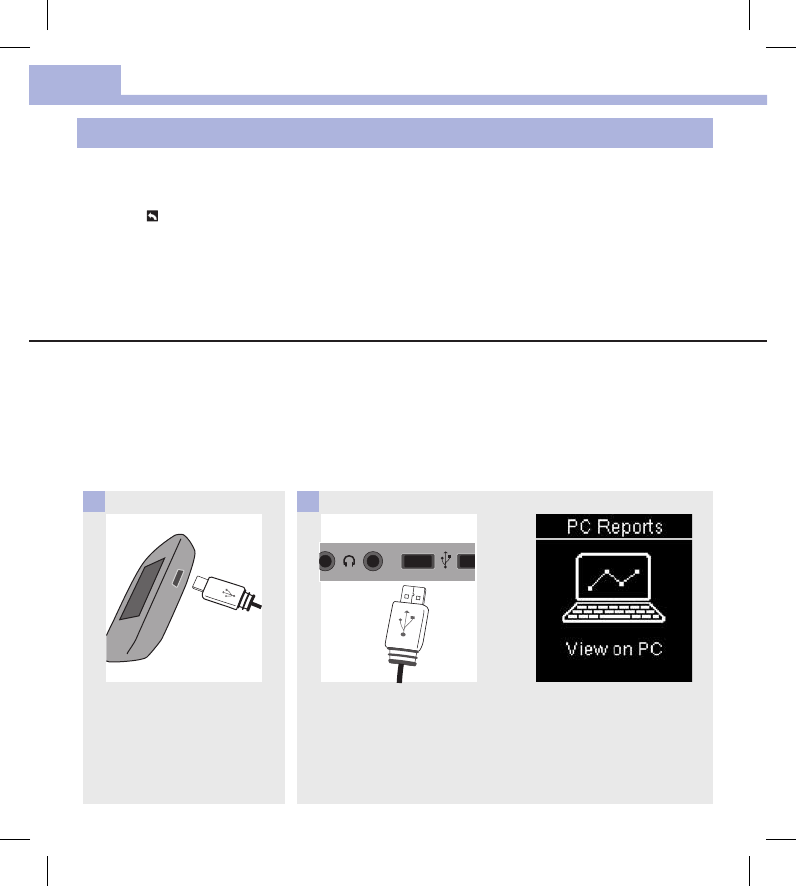
Plug the small end of the USB
cable into the meter.
1
Plug the large end of the USB
cable into a USB port on a PC.
If the meter is o, it turns on
and displays PC Reports.
2
View the Data on a PC (in an Internet Browser)
These steps assume that USB Default is set to PC Reports.
The meter can be o or on.
Once the meter is disconnected from the PC, the data disappears from the PC (but not the meter) unless you
save it on the PC.
52195_AvivaConnect_FDA.indb 74 5/1/14 7:23 AM

Data Transfer and PC Reports
75
8
Double click on the ACCU‑CHEK
drive icon.
4
Click on Start.html.
The Internet browser opens and
the default reports appear.
5
NOTE
If you would like to view the data in special diabetes management software instead, follow these steps:
1. Unplug the USB cable. Connection Lost appears.
2. Press to return to Main Menu.
3. Select My Data>Data Transfer>USB Cable.
4. Reconnect the USB cable.
5. Data Transfer appears.
Open the file manager (for
example, Microsoft Windows
Explorer) on the PC.
The meter appears as a drive
(USB storage device) in the file
manager.
3
52195_AvivaConnect_FDA.indb 75 5/1/14 7:23 AM

Data Transfer and PC Reports
76
8
Shortcut
Here is a shortcut to get to Data Transfer or PC Reports. This is a quick way to transfer the data to software
or to view the meter’s data on a PC.
1. Turn the meter off.
2. Press and hold both and until Activity appears.
3. Select either Data Transfer or PC Reports.
View PC Reports
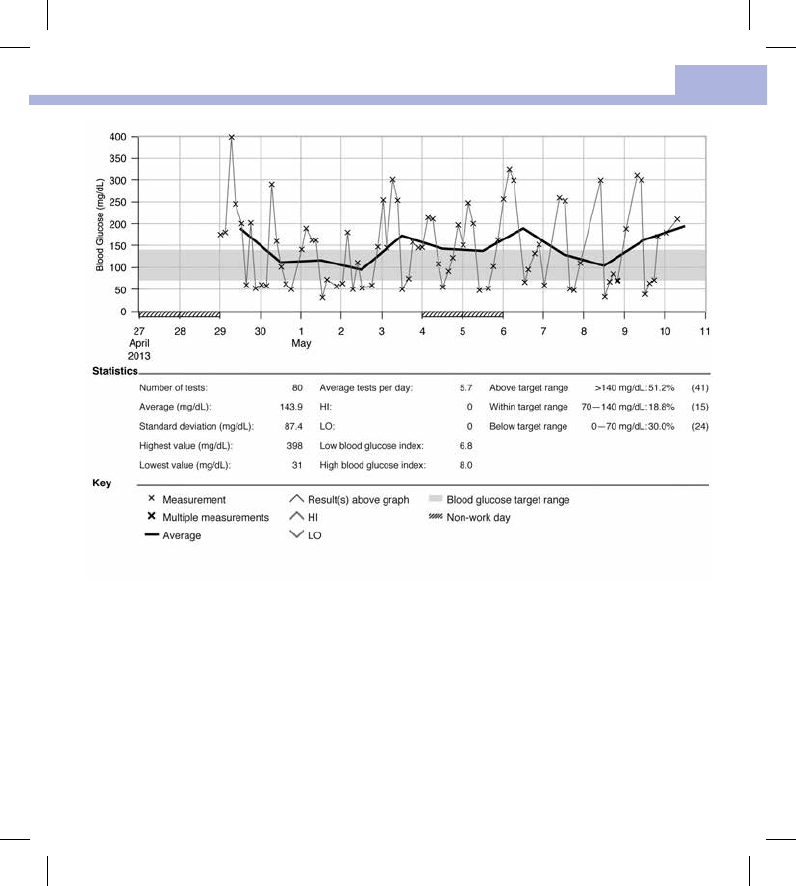
Overview
• An Internet connection is not necessary to view PC Reports.
• When the meter and PC successfully connect, the following reports are displayed in the Internet browser:
• Trend Report – This report shows the trend for several test results over the selected time period (past
3, 7, 14, 30, or 90days).
• Standard Day Report – This report shows all data in a 24‑hour grid.
• Standard Week Report – This report shows all blood glucose results according to the time when the
test was performed and the day of the week.
• List Report – The list report (record list) shows the test results sorted by date and time of the test.
52195_AvivaConnect_FDA.indb 76 5/1/14 7:23 AM
Data Transfer and PC Reports
77
8
52195_AvivaConnect_FDA.indb 77 5/1/14 7:23 AM
Data Transfer and PC Reports
78
8
Statistics
Below the chart of a report, you will find a statistical analysis of all test results plotted with the following
information:
• Number of tests
• Average
• Standard deviation – The standard deviation is the variance of the analyzed results.
• Highest value
• Lowest value
• Average tests per day – Average number of blood glucose tests per day
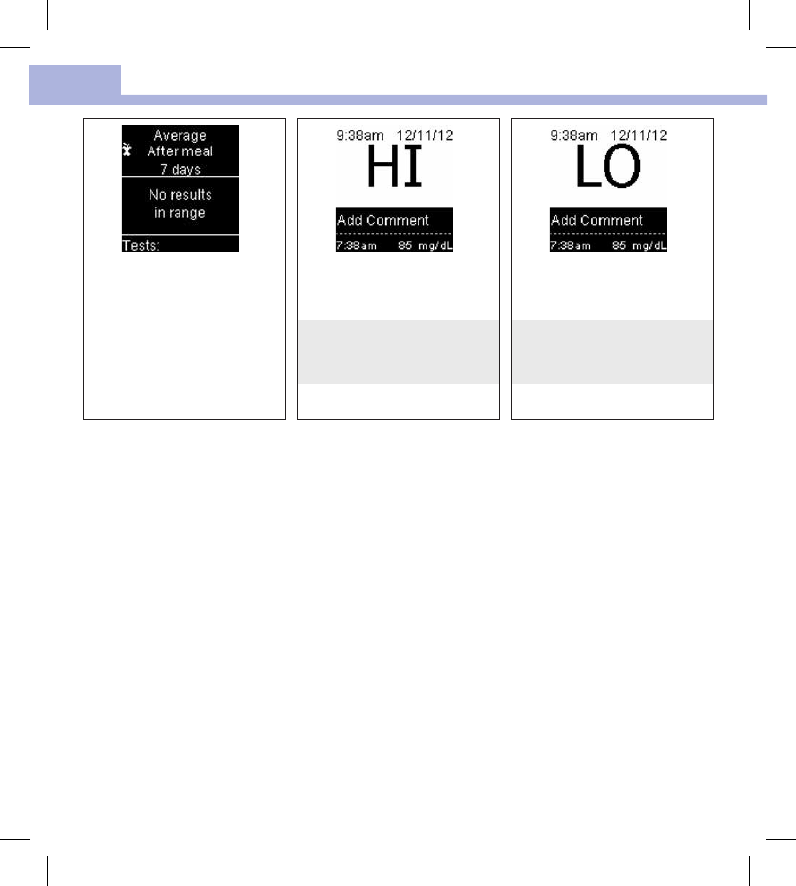
• HI or LO – Blood glucose results outside the measuring range
• Low blood glucose index or High blood glucose index – Further information can be found in References
• Above target range – Blood glucose results above the target range
• Within target range – Blood glucose results within the target range
• Below target range – Blood glucose results below the target range
Key
x Blood glucose result
X Several blood glucose results
––– Average blood glucose results in the selected time period
^ Blood glucose result above the chart range
^ (red caret) Blood glucose may be higher than the measurement range of the system
∨Blood glucose may be lower than the measurement range of the system
(green) Your personal blood glucose target range (shown as a green bar on the PC monitor)
(black) Non‑work days
52195_AvivaConnect_FDA.indb 78 5/1/14 7:23 AM
Data Transfer and PC Reports
79
8
Print Report
Do not use the print function of the Internet browser. Use the Print reports button instead.
Excluded Data
The following blood glucose results are not included in a report:
• Test results outside the selected time period
• Control results
• LO or HI test results
52195_AvivaConnect_FDA.indb 79 5/1/14 7:23 AM
Data Transfer and PC Reports
80
8
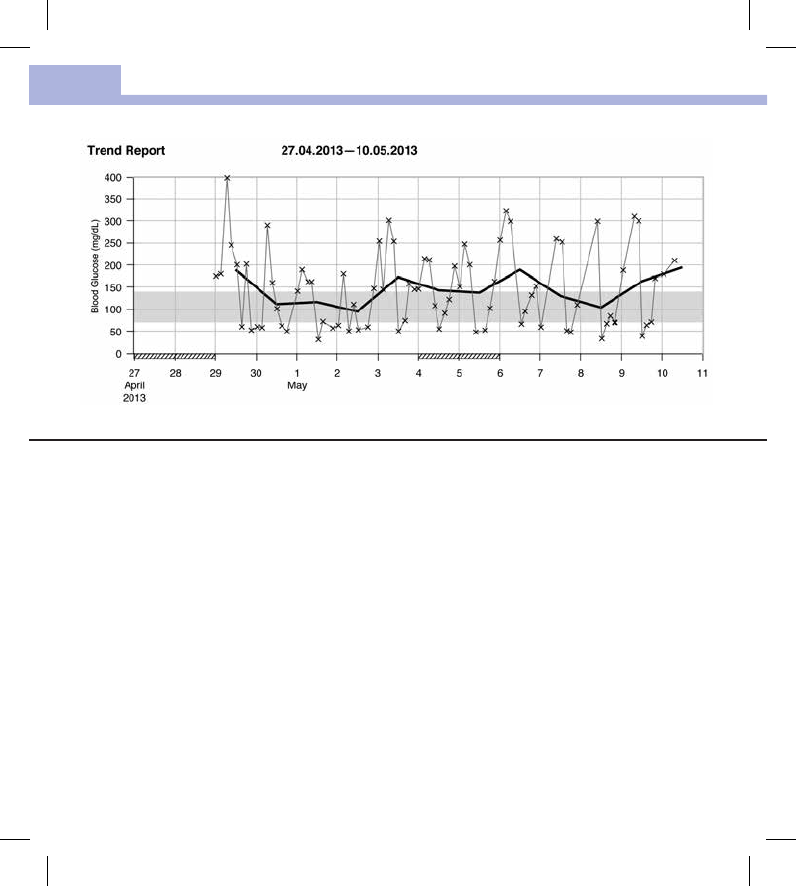
The Trend Report shows the trend of blood glucose results over the selected time period.
The date appears on the horizontal x‑axis. The blood glucose results appear on the vertical y‑axis. The test
results are connected by a thin black line in chronological order.
If you set a target range in the meter, it appears as a green bar on the chart. Non‑work days are marked
with diagonal slashes on the horizontal x‑axis.
The trend of the day‑to‑day average blood glucose result is represented by a thick black line.
52195_AvivaConnect_FDA.indb 80 5/1/14 7:23 AM
Data Transfer and PC Reports
81
8
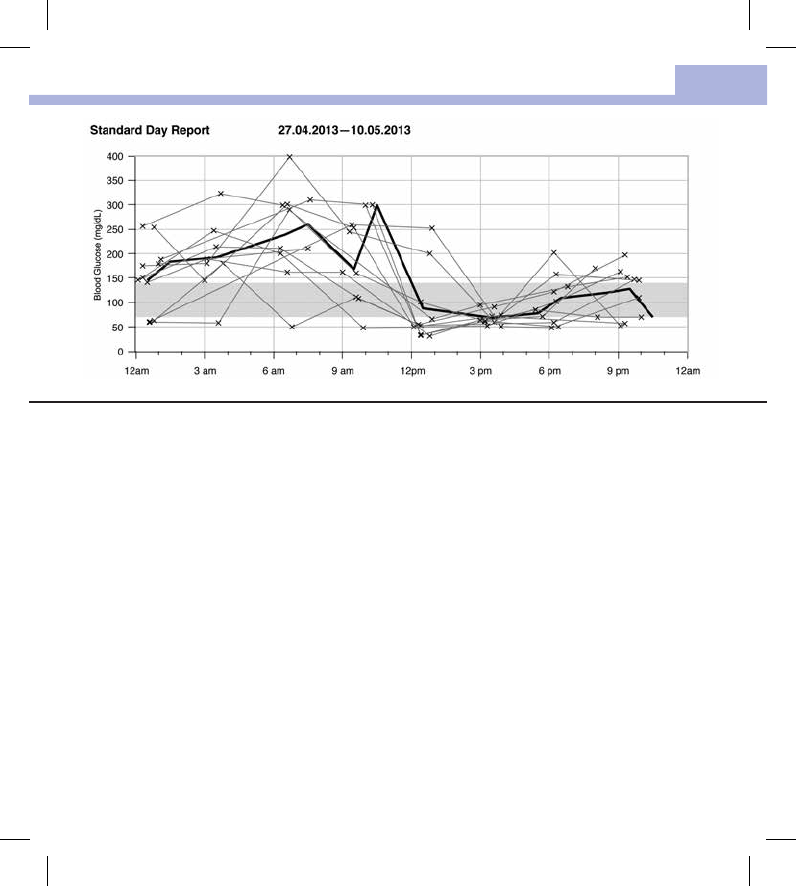
The Standard Day Report makes it easier to recognize daily patterns. All blood glucose results are placed on a
24‑hour grid. Therefore, all tests performed at (approximately) the same time are shown at the same position
on the horizontal time axis.
Blood glucose results are connected by a thin black line in chronological order. A thick black line represents
the trend of the average level (in intervals of 1hour if a test result falls in each interval).
52195_AvivaConnect_FDA.indb 81 5/1/14 7:23 AM
Data Transfer and PC Reports
82
8
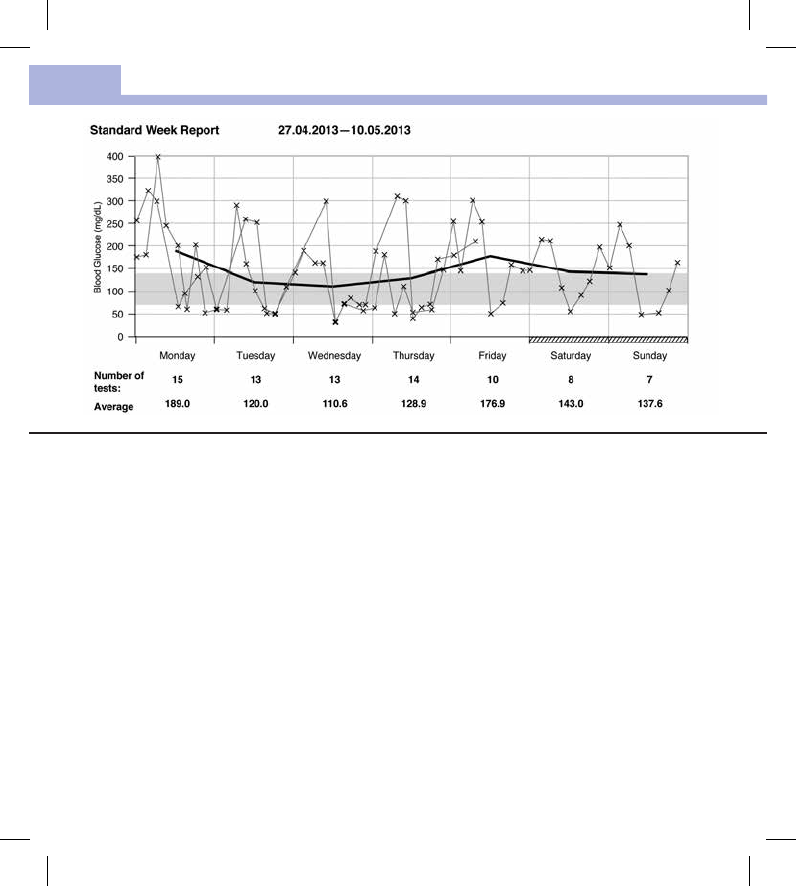
The Standard Week Report makes it easier to recognize weekly patterns. For example, you might find trends
brought about by your occupation.
All blood glucose results are plotted on the chart according to the time and day the test was performed.
Blood glucose results are connected by a thin black line in chronological order. A thick black line represents
the trend of the average level for each day.
The number of tests and the daily blood glucose average are listed below the chart.
52195_AvivaConnect_FDA.indb 82 5/1/14 7:23 AM
Data Transfer and PC Reports
83
8
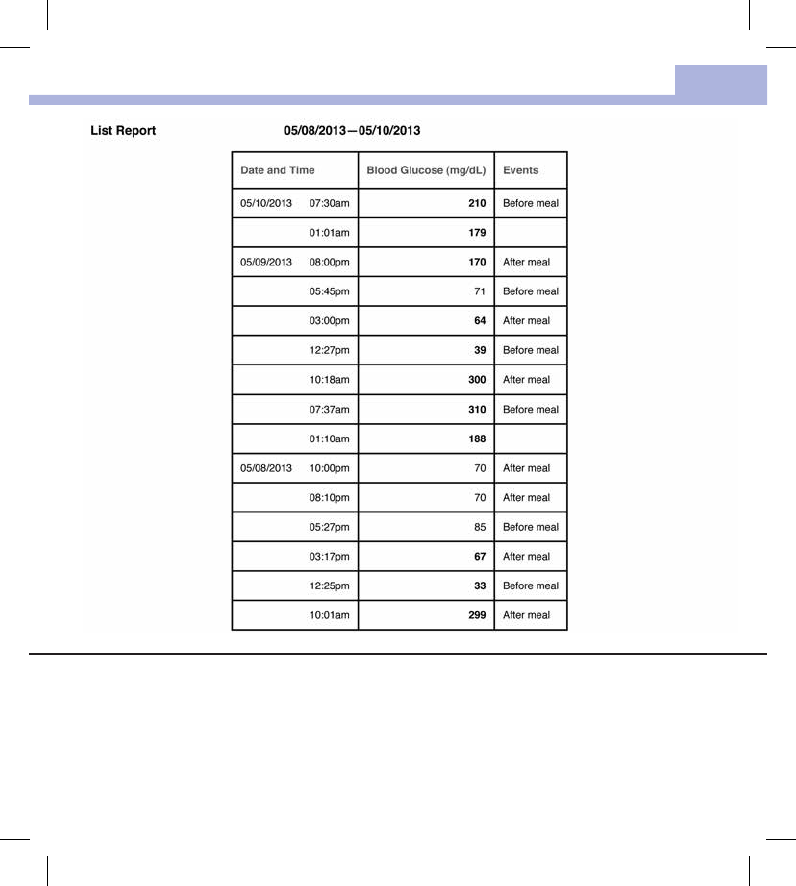
The List Report shows the test results sorted by date and time of the test. All blood glucose results are listed
chronologically with any additional information about the test result.
The list contains the following columns:
• Date and Time
• Blood Glucose
• Events – Event connected to this test result
52195_AvivaConnect_FDA.indb 83 5/1/14 7:23 AM
Data Transfer and PC Reports
84
8
Working with Reports
Analyzing Data in External Applications
If you want to analyze the test results using external software, you can save the data as a CSV file (Comma
Separated Values). CSV files can be opened with a text editor or spreadsheet program.
The CSV file contains all test results saved in the meter. Test results transferred at an earlier date are
transferred again.
1. Press the Save file button on the user interface.
Depending on the configuration of the PC, the CSV file may be directly opened in a spreadsheet
program. In this case, you can save the data using the Save function of the spreadsheet program.
2. In the dialog box that opens, select the option to save the file.
Where you find the CSV file on the PC depends on the settings in the operating system for data
download.
The CSV file contains the following information:
• Serial number: Serial number of the meter.
• Download date, download time: date and time when the meter transferred the test results to the
computer.
• Date, time, result and unit of the results saved in the meter.
• Flags added to the results, indicated by an X.
In the CSV file, the date is always displayed as DD.MM.YYYY and the time as 24‑hour format (hh:mm). The
time format set in the meter has no influence on the format of the date and time in the CSV file.
Security Settings within the Internet Browser
The settings of the Internet browser can influence working with reports. The reports use pages with active
content (JavaScript). This active content can be suppressed by security settings in the browser, causing
warnings or restricted functionality. If this happens, check the Internet browser settings.
In many cases, you can create dierent security settings for using the Internet and working with reports (for
example, at user login to the PC or by defining user profiles in the browser).
If you select the Internet browser security settings appropriately (for example, Allow active content to run
in files on My Computer), you can work with reports without any restrictions.
52195_AvivaConnect_FDA.indb 84 5/1/14 7:23 AM
Data Transfer and PC Reports
85
8
NOTE
Some newer Internet browsers do not allow the Save function (e.g., Mozilla Firefox version 15 and higher).
The files can be saved from Windows Explorer and subsequently opened using programs that are compatible
with *.CSV files.
Troubleshooting
Troubleshooting Check Action
The ACCU‑CHEK drive symbol with the start.html
file does not appear on the PC.
Check whether PC Reports is selected as the
default.
Check whether the PC or operating system
supports data transfer via USB.
Check whether the USB connector is firmly plugged
into the correct port on the PC.
The meter is still not detected as a drive. Plug the meter into a dierent USB port on the PC.
52195_AvivaConnect_FDA.indb 85 5/1/14 7:23 AM

Data Transfer and PC Reports
86
8
52195_AvivaConnect_FDA.indb 86 5/1/14 7:23 AM

Meter and Lancing Device Cleaning and Disinfecting
87
9
What is the dierence between cleaning and disinfecting?
Cleaning is the removal of dirt from the meter or lancing device.
3
Disinfecting is the removal of most, but not all, disease‑causing and other types of microorganisms
(bloodborne pathogens) from the meter or lancing device.
3
Approved Cleaning and Disinfecting Product
The following product has been approved for cleaning and disinfecting the meter and lancing device:
Super Sani‑Cloth (EPA*reg.no.9480‑4)
Super Sani‑Cloth can be purchased from Amazon.com, Ocedepot.com, and Walmart.com.
• Do not use any other cleaning or disinfecting solutions. Using solutions other than the Super Sani‑Cloth
could result in damage to the meter and lancing device.
• The eect of using more than one product interchangeably to clean and disinfect the meter and lancing
device has not been tested. Always use Super Sani‑Cloth to clean and disinfect the meter and lancing
device.
• Roche has tested the approved product for a total of 260disinfection cycles, which is equal to disinfecting
once per week over a 5year period.
*Environmental Protection Agency
Chapter 9: Meter and Lancing Device Cleaning and Disinfecting
52195_AvivaConnect_FDA.indb 87 5/1/14 7:23 AM
Meter and Lancing Device Cleaning and Disinfecting
88
9
NOTE
For technical assistance or questions on cleaning and disinfecting, contact the ACCU‑CHEK Customer Care
Service Center at 1‑800‑858‑8072.
Cleaning and Disinfecting the Meter
WARNING
If the meter is being operated by a second person who is providing testing assistance to the user, the
meter and lancing device should be cleaned and disinfected prior to use by the second person.
To clean and disinfect without damaging the meter, follow these procedures carefully.
When to Clean and Disinfect the Meter
• Clean the meter to remove visible dirt or other material prior to disinfecting.
• Clean and disinfect the meter at least once per week and when blood is present on the surface of the
meter.
• Clean and disinfect the meter before allowing anyone else to handle the meter. Do not allow anyone
else to use the meter on themselves for testing purposes.
NOTE
Using cleaning and disinfecting products could result in damage to the meter. If you notice any of the
following signs of deterioration after cleaning and disinfecting your meter, stop using your meter and contact
the ACCU‑CHEK Customer Care Service Center at 1‑800‑858‑8072: residue around buttons, clouding of
display, button malfunction, out‑of‑range control results.
What to Clean and Disinfect
The following parts of the meter should be cleaned and disinfected:
• The area around slots and openings (do not get any moisture in slots or openings)
• The meter display
• The entire meter surface
52195_AvivaConnect_FDA.indb 88 5/1/14 7:23 AM
Meter and Lancing Device Cleaning and Disinfecting
89
9
How to Clean and Disinfect the Meter
WARNING
Failure to follow these instructions will damage the meter and stop it
from working properly.
• DO NOT clean or disinfect the meter while performing a blood glucose
or control test.
• DO NOT get any moisture in slots or openings.
• DO NOT spray anything onto the meter.
• DO NOT immerse the meter in liquid.
• Always use the same product for both cleaning and disinfecting.
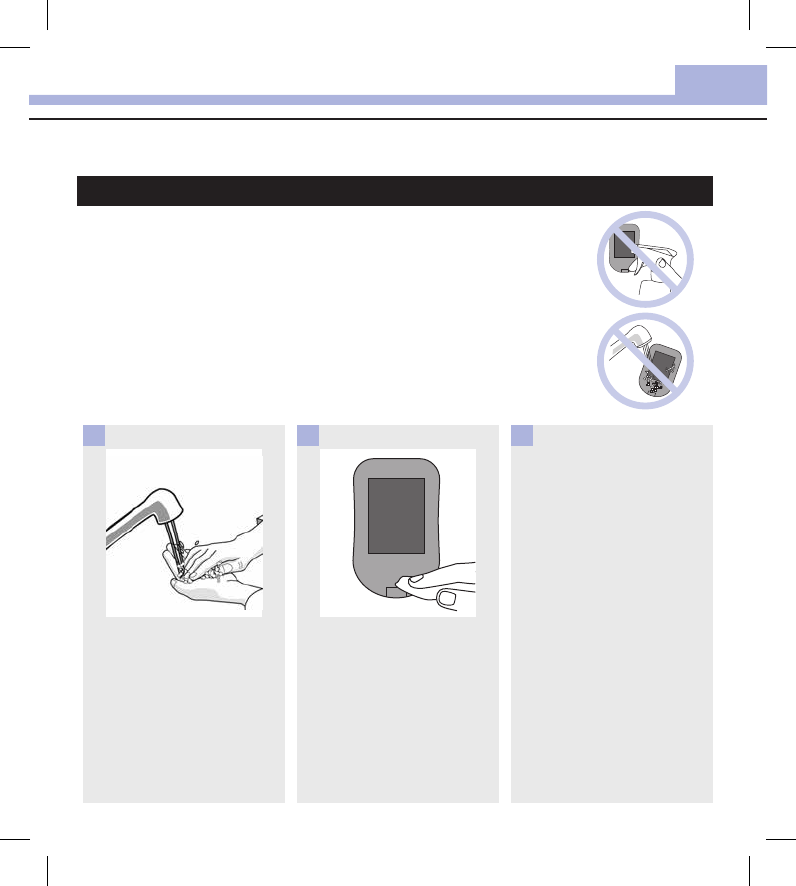
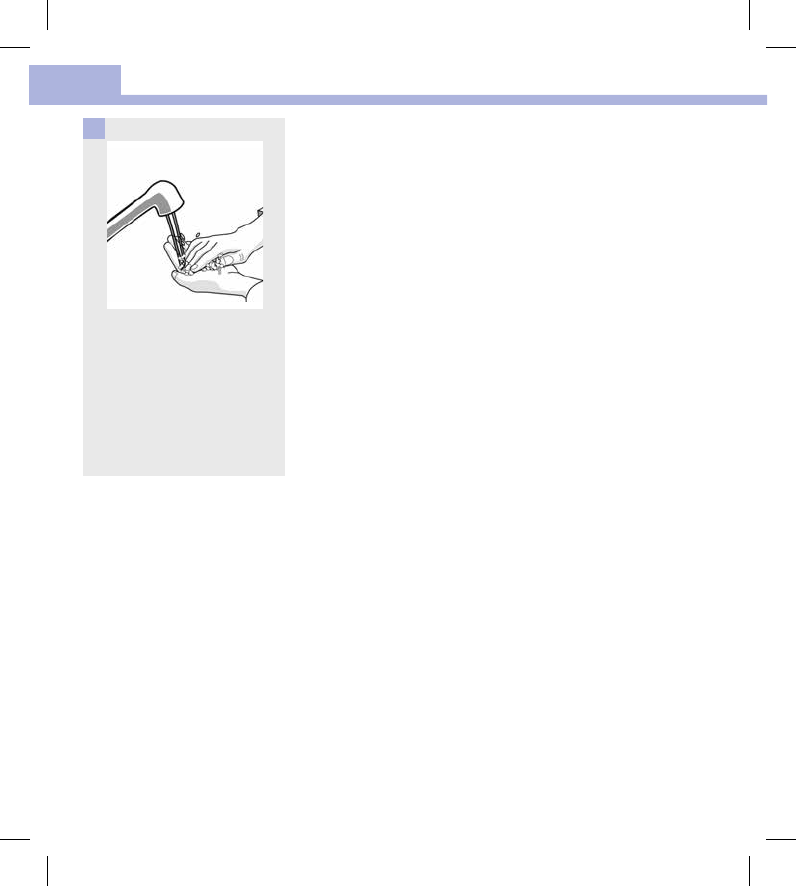
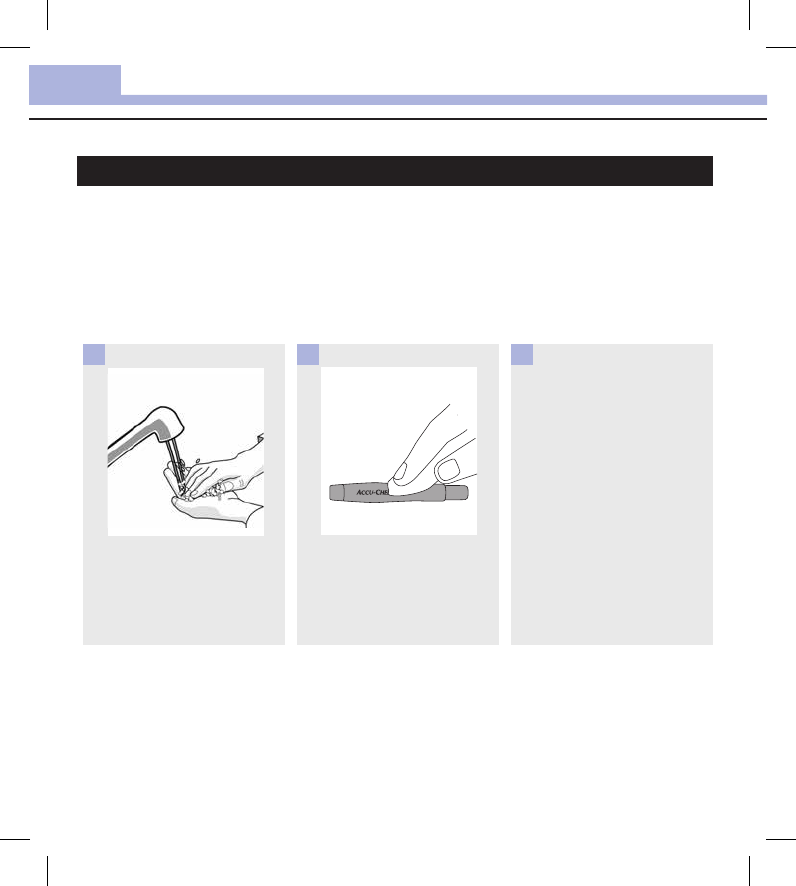
Wash hands thoroughly with
soap and water.
1
Turn the meter off and wipe the
entire meter surface with a
Super Sani‑Cloth. Carefully wipe
around the test strip slot and
other openings.
Make sure that no liquid enters
any slot or opening.
2
A separate Super Sani‑Cloth
should be used for cleaning and
disinfection. For disinfecting the
meter, get a new cloth and
repeat step2, making sure the
surface stays wet for 2minutes.
Make sure that no solution is
seen in any slot or opening.
3
52195_AvivaConnect_FDA.indb 89 5/1/14 7:23 AM
Meter and Lancing Device Cleaning and Disinfecting
90
9
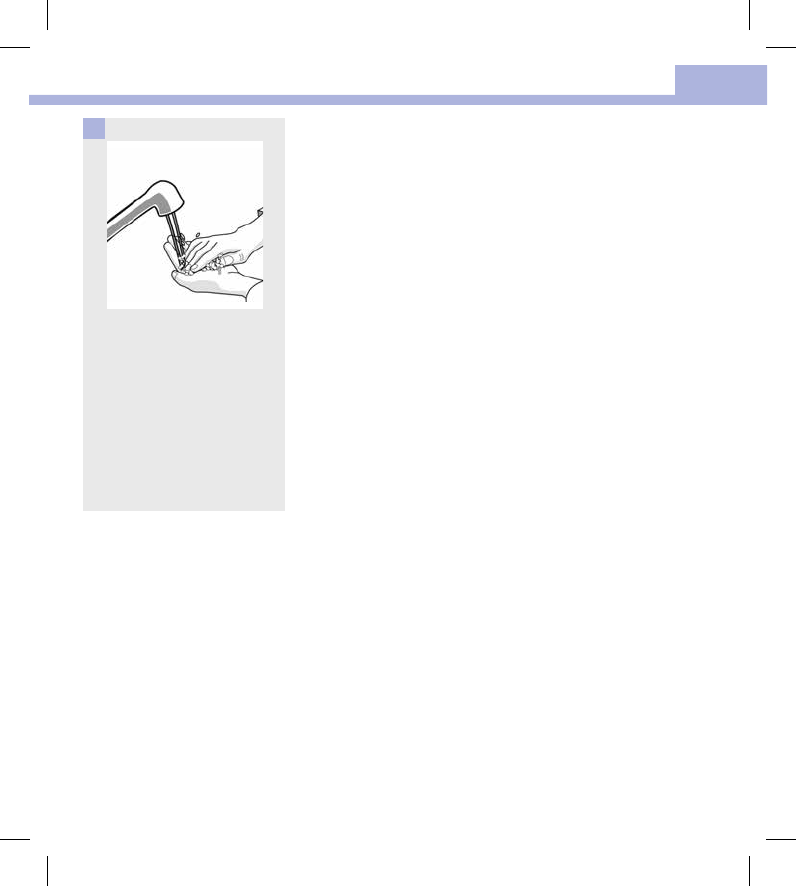
Wash hands thoroughly with
soap and water.
4
52195_AvivaConnect_FDA.indb 90 5/1/14 7:23 AM
Meter and Lancing Device Cleaning and Disinfecting
91
9
Cleaning and Disinfecting the Lancing Device
To clean and disinfect without damaging the lancing device, follow these procedures carefully.
When to Clean and Disinfect the Lancing Device
• Clean the lancing device to remove visible dirt or other material prior to disinfecting.
• Clean and disinfect the lancing device at least once per week to remove visible dirt or other material
for safe handling.
• Clean and disinfect the lancing device before allowing anyone else to handle the lancing device, for
instance, if you have someone assisting you. Do not allow anyone else to use the lancing device.
NOTE
• Do not throw away the cap after each use. Use the approved cleaning and disinfecting product on the
cap.
• Always remove the lancet drum before cleaning or disinfecting the lancing device.
• Using cleaning and disinfecting products could result in damage to the lancing device. If you notice any
of the following signs of deterioration after cleaning and disinfecting your lancing device, stop using
your lancing device and contact the ACCU‑CHEK Customer Care Service Center at 1‑800‑858‑8072:
residue around buttons, diculty in priming the device, diculty in inserting the lancet drum.
• You might observe a slight discoloration of the lancing device after multiple cleaning and disinfecting
cycles. This does not aect the functionality of the lancing device.
What to Clean and Disinfect
The following parts of the lancing device should be cleaned and disinfected:
• The entire lancing device surface
• The cap
52195_AvivaConnect_FDA.indb 91 5/1/14 7:23 AM
Meter and Lancing Device Cleaning and Disinfecting
92
9
How to Clean and Disinfect the Lancing Device
WARNING
Failure to follow these instructions may damage the lancing device and stop it from working properly.
• DO NOT get any moisture into any openings.
• Always use the same product for both cleaning and disinfecting.
Wash hands thoroughly with
soap and water.
1
Wipe the entire surface of the
lancing device and the inside of
the cap with an approved
cleaning and disinfecting
product.
2
A separate Super Sani‑Cloth
should be used for cleaning and
disinfection. For disinfecting the
lancing device, use a new cloth
and repeat step2 making sure
the surface stays wet for
2minutes.
3
52195_AvivaConnect_FDA.indb 92 5/1/14 7:23 AM
Meter and Lancing Device Cleaning and Disinfecting
93
9
Wash hands thoroughly with
soap and water.
4
52195_AvivaConnect_FDA.indb 93 5/1/14 7:23 AM

Meter and Lancing Device Cleaning and Disinfecting
94
9
52195_AvivaConnect_FDA.indb 94 5/1/14 7:23 AM
Meter Maintenance and Troubleshooting
95
10
Meter Maintenance
The meter automatically tests its own systems every time you turn it on and lets you know if something is
wrong. See Error Messages in this chapter.
If you have problems with the meter or think the results are not accurate, perform a control test with an
unexpired test strip and control solution. If the control result is not within the acceptable range, contact the
ACCU‑CHEK Customer Care Service Center at 1‑800‑858‑8072.
Changing the Batteries
Chapter 10: Meter Maintenance and Troubleshooting
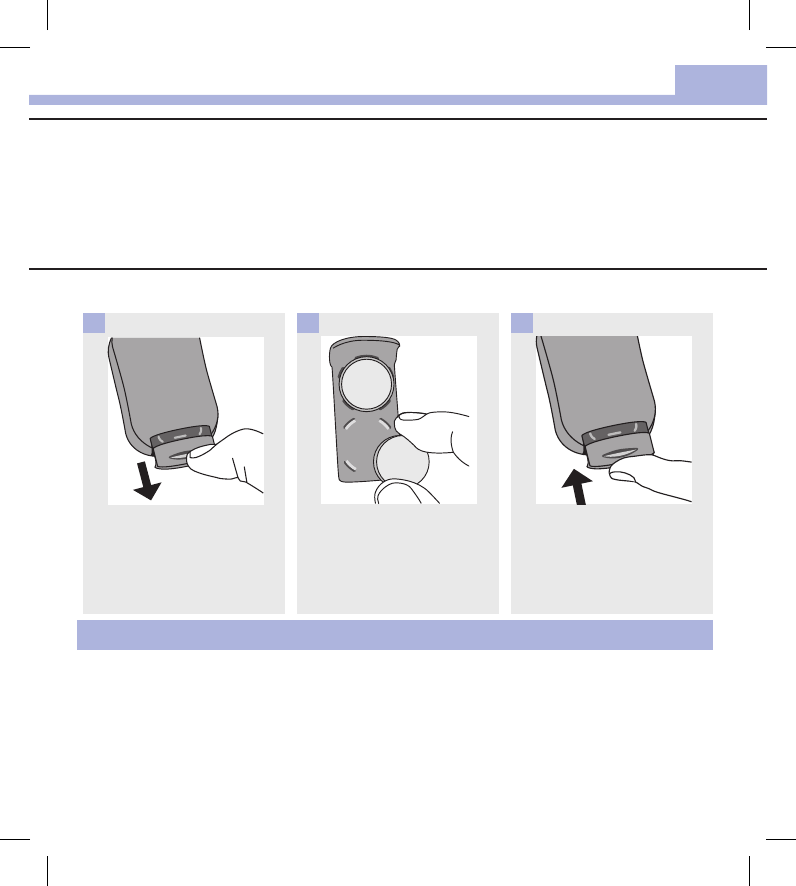
Use your thumb to slide the
battery drawer out of the meter.
1
Remove the old batteries and
place the new ones in the
battery drawer with the (+)side
facing down.
2
Slide the battery drawer back
into position until it locks into
place.
3
NOTE
• The meter uses two3‑volt lithium batteries, coin cell typeCR2032. This type of battery can be found in
many stores. It is a good idea to have spare batteries available.
• Always replace both batteries at the same time and with the same brand.
• The logbook data is saved when you replace the batteries.
52195_AvivaConnect_FDA.indb 95 5/1/14 7:23 AM

Meter Maintenance and Troubleshooting
96
10
Error Messages
WARNING
Never make therapy decisions based on an error message.
The meter is connected to a PC
and a test cannot be performed.
EITHER remove the USB cable
and perform a test OR remove
the test strip and start a data
transfer.
The meter will not turn on or the
display is blank.
Batteries are dead.
Insert new batteries.
Display is damaged. / Meter is
defective.
Contact the ACCU‑CHECK
Customer Care Service Center
at 1‑800‑858‑8072.
Extreme temperatures.
Move the meter to a more
temperate area.
The connection between the
meter and PC was lost.
Disconnect and reconnect the
USB cable and retry the
connection. Contact the
ACCU‑CHEK Customer Care
Service Center if the connection
is lost again.
52195_AvivaConnect_FDA.indb 96 5/1/14 7:23 AM
Meter Maintenance and Troubleshooting
97
10
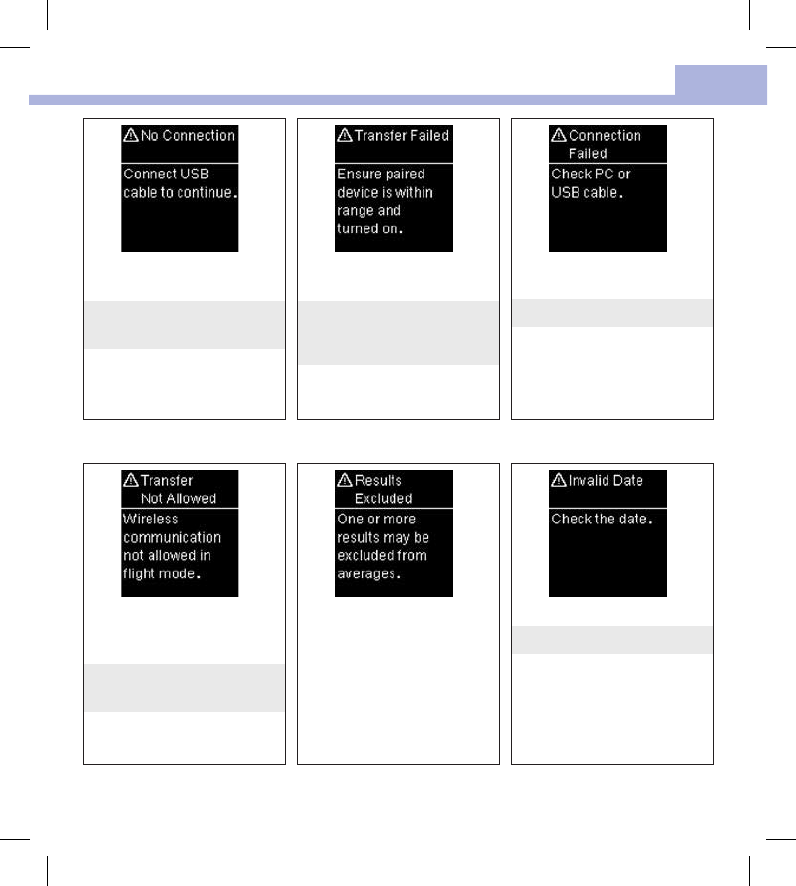
There is no connection between
the meter and PC.
Connect the USB cable and retry
the connection.
Blood glucose results were not
transferred to a paired device.
Make sure the paired device is
within range of the meter and
turned on.
Data could not be transferred
from the meter to the PC.
Check the PC or USB cable.
Data cannot be sent to a paired
device because the meter is in
Flight Mode.
Retry the data transfer when the
meter is not in Flight Mode.
One or more blood glucose
results are excluded from the
selected averages because the
results are invalid or out of the
system measurement range.
The date entered is not valid.
Enter the correct date.
52195_AvivaConnect_FDA.indb 97 5/1/14 7:23 AM
Meter Maintenance and Troubleshooting
98
10
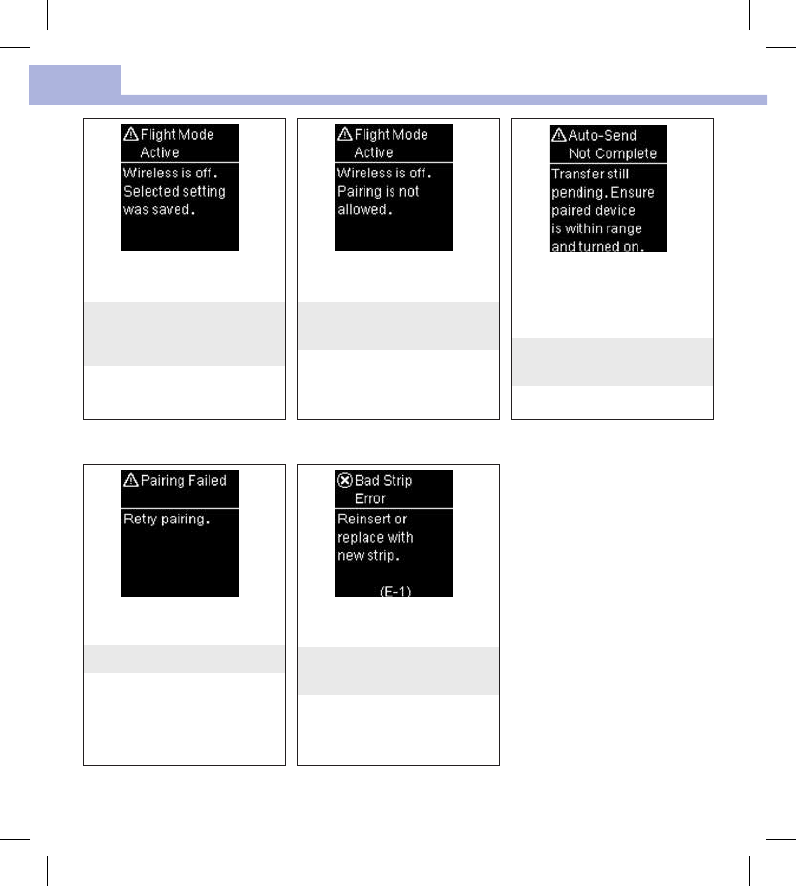
A meter setting was changed
while in Flight Mode.
The setting change will not take
eect until Flight Mode is
turned o.
Pairing to a device cannot be
performed while in Flight Mode.
Retry pairing when the meter is
not in Flight Mode.
The blood glucose result has not
been sent to the default paired
device. The transfer is still
pending.
Place meter and paired device
closer together.
The meter was unable to pair
with a device.
Retry the pairing.
The test strip may be damaged
or not properly inserted.
Remove and reinsert the test
strip or replace it if damaged.
52195_AvivaConnect_FDA.indb 98 5/1/14 7:23 AM

Meter Maintenance and Troubleshooting
99
10
and above the system’s
reading range. Contact your
healthcare professional
immediately.
• If the second test result does
not match how you feel,
perform a control test with
the control solution and a
new test strip.
• If the control result is
within the acceptable
range, review the proper
testing procedure and
repeat the blood glucose
test with a new test strip.
• If the control result
is not within the
acceptable range, see
the Understanding
Out‑of‑Range Control
Results section in the
chapter Control Tests.
Your blood glucose may be
extremely high or a meter or a
test strip error has occurred.
• If your test result matches
how you feel, contact your
healthcare professional
immediately.
• If your test result does not
match how you feel, repeat
the blood glucose test. See
the Unusual Blood Glucose
Results section in the chapter
Blood Glucose Tests.
• If the E‑3code still appears
for your blood glucose test,
your blood glucose result
may be extremely high
A meter or test strip error has
occurred.
52195_AvivaConnect_FDA.indb 99 5/1/14 7:23 AM
Meter Maintenance and Troubleshooting
100
10
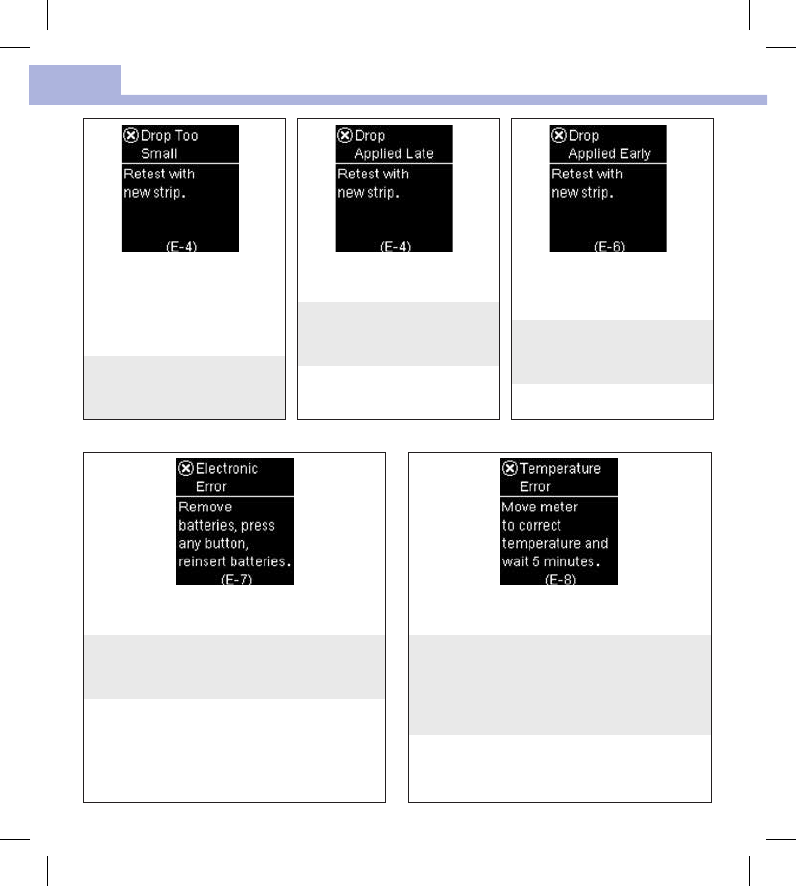
Not enough blood or control
solution was drawn into the test
strip for measurement or was
applied after the test had
started.
Discard the test strip and repeat
the blood glucose or control
test.
The blood or control drop was
applied to the test strip too late.
Discard the test strip and repeat
the blood glucose or control
test.
Blood or control solution was
applied to the test strip before
Apply drop appeared.
Discard the test strip and repeat
the blood glucose or control
test.
An electronic error occurred, or in rare cases, a
used test strip was removed and reinserted.
Remove the batteries, press any button, and
reinsert the batteries. Perform a blood glucose or
control test.
The temperature is above or below the proper
range for the system.
Refer to the test strip package insert for system
operating conditions. Move to an area with the
appropriate conditions, wait 5minutes, and
repeat the blood glucose or control test. Do not
artificially heat or cool the meter.
52195_AvivaConnect_FDA.indb 100 5/1/14 7:23 AM
Meter Maintenance and Troubleshooting
101
10
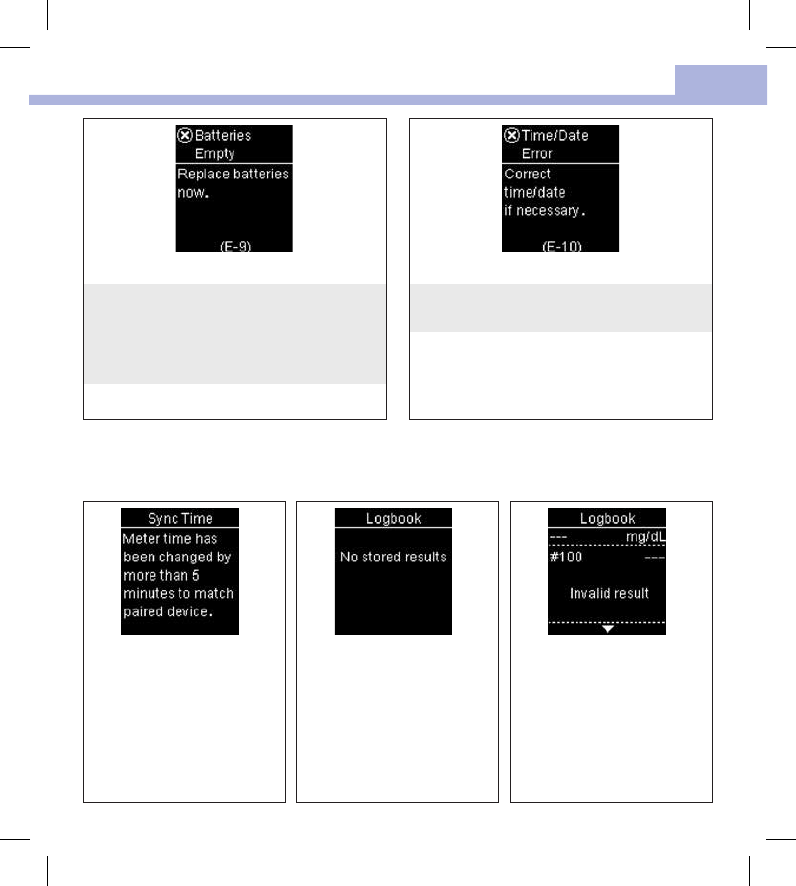
The batteries are out of power.
Change the batteries now. If the message
reappears after the batteries have been replaced,
slide the battery drawer out of the meter, press
any meter button, then reinsert the battery
drawer into position.
The time and date setting may be incorrect.
Make sure the time and date are correct and
adjust, if necessary.
The meter time and date have
been changed to match the
paired device.
There are no results in the
Logbook.
There is an invalid result in the
Logbook.
52195_AvivaConnect_FDA.indb 101 5/1/14 7:24 AM
Meter Maintenance and Troubleshooting
102
10
There are no results in range for
the selected average.
Blood glucose may be higher
than the measurement range of
the system.
See the Unusual Blood Glucose
Results section in the chapter
Blood Glucose Tests.
Blood glucose may be lower
than the measurement range of
the system.
See the Unusual Blood Glucose
Results section in the chapter
Blood Glucose Tests.
52195_AvivaConnect_FDA.indb 102 5/1/14 7:24 AM

Technical Information
103
11
Product Limitations
See the literature packaged with the test strips and control solutions for the latest information on product
specifications and limitations.
Specifications
Blood volume
Sample type
Measuring time
Measurement range
Test strip storage conditions
System operating conditions
Refer to the test strip package insert.
Meter storage conditions Temperature: ‑13–158°F
Memory capacity 750blood glucose results and 30control results with
time and date
Automatic o 90seconds
Power supply Two3‑volt lithium batteries (coin cell type CR2032)
Display LCD
Dimensions 80 × 47 × 19 mm (LWH)
Weight Approx. 40g (with batteries)
Construction Hand‑held
Protection class III
Meter type The ACCU‑CHEK Aviva Connect meter is suitable for
continuous operation.
Control solution storage conditions Refer to the control solution package insert.
Chapter 11: Technical Information
52195_AvivaConnect_FDA.indb 103 5/1/14 7:24 AM

Technical Information
104
11
Bluetooth
®
Wireless Technology
The meter uses Bluetooth smart class II wireless technology to communicate and transfer information.
Bluetooth wireless technology is a form or radio frequency (RF) technology that operates in the unlicensed
industrial, scientific and medical band at 2.4 to 2.485 GHz. The RF channel utilized for communication
between the meter and other devices, such as a smartphone, is not an open channel. The meter can only
communicate with the device that
1. is Bluetooth smart,
2. it is paired with, and
3. has an application that can accept the meter’s data.
This device complies with United States Federal Communication Commission (FCC) standards. The device
complies with FCC Part 15 Rules. Operation of the device is subject to the following conditions:
1. This device may not cause harmful interference and
2. must accept any interference received, including interference that may cause undesired operation.
Compliance with these guidelines means that under normal, daily circumstances, the device should not
aect the operation of other devices. In addition, the device should operate normally in the presence of other
devices.
In the event there is interference from another device, it is recommended that you increase the distance
between the meter and that device. You can also turn o the interfering device. In addition, you can turn o
Bluetooth wireless technology on the meter. Changes or modifications to the device not expressly approved
by Roche could void the user’s authority to operate the device. The device has been tested and found to
comply with the limits for a Class B digital device. The device generates, uses, and can radiate radio
frequency energy.
52195_AvivaConnect_FDA.indb 104 5/1/14 7:24 AM

Technical Information
105
11
Electromagnetic Compatibility – This meter meets the electromagnetic immunity requirements as per
ENISO15197. The chosen basis for electrostatic discharge immunity testing was basic standard
IEC61000‑4‑2. In addition, the meter meets the electromagnetic emissions requirements as per EN61326.
The meter’s electromagnetic emission is thus low. Interference from the meter to other electrically‑driven
equipment is not anticipated.
This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions:
(1) This device may not cause harmful interference, and (2) this device must accept any interference
received, including interference that may cause undesired operation.
This equipment complies with FCC and IC radiation exposure limits set forth for an uncontrolled environment.
This equipment is in direct contact with the body of the user under normal operating conditions. This
transmitter must not be co‑located or operating in conjunction with any other antenna or transmitter.
NOTE
This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to
Part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful
interference in a residential installation. This equipment generates, uses and can radiate radio frequency
energy and, if not installed and used in accordance with the instructions, may cause harmful interference to
radio communications. However, there is no guarantee that interference will not occur in a particular
installation. If this equipment does cause harmful interference to radio or television reception, which can be
determined by turning the equipment o and on, the user is encouraged to try to correct the interference by
one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit dierent from that to which the receiver is connected.
• Consult the dealer or an experienced radio/TV technician for help.
Changes or modifications not expressly approved by the party responsible for compliance (i.e. the
manufacturer) could void the user’s authority to operate the equipment.
Performance Analysis – Refer to the test strip package insert.
Test Principle – Refer to the test strip package insert.
52195_AvivaConnect_FDA.indb 105 5/1/14 7:24 AM
Technical Information
106
11
Product Safety Information
WARNING
• This meter meets IEC 61010‑1, IEC61010‑101 and IEC 609501‑1 safety standards.
• Strong electromagnetic fields may interfere with the proper operation of the meter. Do not use the meter
close to sources of strong electromagnetic radiation.
• To avoid electrostatic discharge, do not use the meter in a very dry environment, especially one in which
synthetic materials are present.
Travel Documentation
If you are traveling on a commercial airline, you may be required to provide documentation certifying that
this meter meets environmental conditions and test procedures for Airborne Equipment (RTCA DO‑160)
section21 Emission of Radio Frequency Energy. Visit accu‑chek.com or contact the ACCU‑CHEK Customer
Care Service Center at 1‑800‑858‑8072 to obtain a copy of the document.
Discarding the Meter, Test Strips, Lancing Devices, Lancets, and Batteries
WARNING
• Any product coming in contact with blood is considered contaminated (potentially infectious).*
• During normal testing, any blood glucose meter may come in contact with blood.
• Lancing devices may also be considered sharps. Disposal of sharps is regulated by law in many
jurisdictions.
Roche is committed to recycling and sustainability. Comply with any laws or ordinances relating to the
disposal of sharps and/or contaminated products. Contact your local health department or other appropriate
authorities for proper handling and disposal of used meters, used test strips, used lancets, and used
batteries. Consider the following points when discarding used testing materials:
Consider recycling the meters and batteries at an appropriate facility. Be aware the meter is potentially
hazardous electronics scrap (e‑scrap) and should be disposed of accordingly. The batteries are potentially
hazardous also and should be discarded accordingly.
Disinfect the meter before recycling or discarding.
*29CFR1910.1030 – Bloodborne pathogens
52195_AvivaConnect_FDA.indb 106 5/1/14 7:24 AM
Technical Information
107
11
Explanation of Symbols
Caution, refer to safety‑related notes in the instructions for use accompanying this product.
3‑volt coin cell type CR2032
Additional Supplies
Test Strips: ACCU‑CHEK Aviva Plus test strips
Control Solutions: ACCU‑CHEK Aviva control solutions
Lancets: ACCU‑CHEK FastClix 102‑ct. lancet drums (17‑6 ct. drums)
Low Blood Glucose Index or High Blood Glucose Index
4,5,6
These figures represent the frequency and the resulting risk of blood glucose values being too low or too
high. Figures should be as low as possible.
The following table provides an overview to assess the risk of blood glucose values being too low or too
high:
Risk Low blood glucose index High blood glucose index
minimal ≤1.1 ≤5.0
low 1.1–2.5 5.0–10.0
medium 2.5–5.0 10.0–15.0
high >5.0 >15.0
WARNING
The index values for low blood glucose or high blood glucose in the table are not blood glucose values.
Ask your healthcare professional if you want to change your therapy based on the index values.
52195_AvivaConnect_FDA.indb 107 5/1/14 7:24 AM

Technical Information
108
11
References
1
FDA Public Health Notification: “Use of Fingerstick Devices on More than One Person Poses Risk for
Transmitting Bloodborne Pathogens: Initial Communication, (2010). Update 11/29/2010.” http://www.fda.gov/
MedicalDevices/Safety/AlertsandNotices/ucm224025.htm. Accessed March 20, 2012.
2
CDC Clinical Reminder: “Use of Fingerstick Devices on More than One Person Poses Risk for Transmitting
Bloodborne Pathogens, (2010).” http://www.cdc.gov/injectionsafety/Fingerstick‑DevicesBGM.html. Accessed
March 20, 2012.
3
Healthcare Infection Control Practices Advisory Committee (HICPAC), William A. Rutala, Ph.D., M.P.H., and
David J. Weber, M.D., M.P.H. Centers for Disease Control and Prevention, 2008. “Guideline for Disinfection
and Sterilization in Healthcare Facilities.” Atlanta.
4
Boris P. Kovatchev, Martin Straume, Daniel J. Cox, Leon S. Farhy (2001) “Risk analysis of blood glucose
data: a quantitative approach to optimizing the control of insulin dependent diabetes.”
Journal of Theoretical
Medicine,
3: pp 1‑10.
5
Boris P. Kovatchev, Daniel J. Cox, Anand Kumar, Linda Gonder‑Frederick, William L. Clarke (2003)
“Algorithmic Evaluation of Metabolic Control and Risk of Severe Hypoglycemia in Type 1 and Type 2 Diabetes
Using Self‑Monitoring Blood Glucose Data.”
Diabetes Technology & Therapeutics
, 5(5): pp 817‑828.
6
Boris P. Kovatchev (2006) “Is Glycemic Variability Important to Assessing Antidiabetes Therapies.”
Current
Diabetes Reports
, 6: pp 350‑356.
52195_AvivaConnect_FDA.indb 108 5/1/14 7:24 AM

License and Warranty
109
12
Warranty
ACCU‑CHEK Aviva Connect Meter 30‑day Money‑back Guarantee for Qualifying Consumers
Roche Diagnostics oers qualifying consumers that purchase an ACCU‑CHEK Aviva Connect meter, a 30‑day
money back guarantee. If you are not fully satisfied with your ACCU‑CHEK Aviva Connect meter, contact the
ACCU‑CHEK Customer Care Service Center toll‑free at 1‑800‑858‑8072 to determine whether you qualify to
receive a full refund within 30 days of purchase. If you are covered under Medicare, Medicaid, other federal/
state programs, or private insurance you are NOT eligible for this money‑back oer. Consumers aected by
this exclusion may instead request a dierent ACCU‑CHEK meter/system. The refund will be limited to the
amount paid by you net of any rebates. You must have a copy of the dated itemized purchase receipt and
the original packaging to obtain this refund.
ACCU‑CHEK Aviva Connect Meter Limited 3‑Year Warranty
Roche Diagnostics warrants to the original purchaser of the meter that your ACCU‑CHEK Aviva Connect
meter will be free from defects in materials and workmanship for three years from the date of purchase. If,
during this 3‑year period, the meter does not work properly because of a defect in materials or
workmanship, Roche Diagnostics will replace it with a new ACCU‑CHEK Aviva Connect meter or equivalent
product free of charge. The warranty on the replacement meter will expire on the date of the original
warranty expiration or 90 days after the shipment of a replacement system, whichever period is longer. The
purchaser’s exclusive remedy with respect to the ACCU‑CHEK Aviva Connect meter shall be replacement.
This warranty does not apply to the performance of an ACCU‑CHEK Aviva Connect meter that has been
damaged by accident or has been altered, misused, tampered with, or abused in any way. Roche Diagnostics
will handle meters that show damage or abuse according to its Non‑Warranty Service Policy described on
the following page.
THE ABOVE WARRANTY IS EXCLUSIVE OF ALL OTHER WARRANTIES, AND ROCHE DIAGNOSTICS MAKES NO
OTHER WARRANTIES, EXPRESS OR IMPLIED, INCLUDING WITHOUT LIMITATION, THE IMPLIED WARRANTY OF
MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. IN NO EVENT SHALL ROCHE DIAGNOSTICS BE
LIABLE TO THE PURCHASER OR ANY OTHER PERSON FOR ANY INCIDENTAL, CONSEQUENTIAL, INDIRECT,
SPECIAL, OR PUNITIVE DAMAGES ARISING FROM OR IN ANY WAY CONNECTED WITH THE PURCHASE OR
OPERATION OF THE METER OR ITS PARTS. NO WARRANTY OF MERCHANTABILITY OR FITNESS FOR A
PARTICULAR PURPOSE, IF ANY IS IMPLIED FROM THE SALE OF THE METER, SHALL EXTEND FOR A LONGER
DURATION THAN THREE YEARS FROM THE DATE OF PURCHASE.
Some states do not allow limitations on how long an implied warranty will last or the exclusion of incidental
or consequential damages, so the above limitation and exclusion may not apply to you. This warranty gives
you specific legal rights, which vary from state to state.
Chapter 12: License and Warranty
52195_AvivaConnect_FDA.indb 109 5/1/14 7:24 AM

License and Warranty
110
12
Non‑Warranty Service Policy
Roche Diagnostics Non‑Warranty Service Policy applies to meters where the above warranty has not become
eective, has become inapplicable, or has expired. Roche Diagnostics will replace, at its option, meters
returned to it for a service charge (not to exceed $35).
Replacement will be with the same or similar product. Replacement meters will be warranted for a period of
90 days from shipment under a limited warranty providing for replacement of parts and labor at no charge.
Warranty and Service Instructions
All requests for return of ACCU‑CHEK Aviva Connect meters under the above warranty or service policy must
be made to the ACCU‑CHEK Customer Care Service Center. You will be mailed a return authorization label,
which must be axed to your carton for shipping the system to Roche Diagnostics. Cartons received without
this label will be returned to you at your expense.
Customers experiencing diculties should review the troubleshooting information in Meter Maintenance
and Troubleshooting of this manual. Further inquiries should be directed to the ACCU‑CHEK Customer Care
Service Center.
Be sure to fill out and mail the Warranty Card that comes with the ACCU‑CHEK Aviva Connect system.
52195_AvivaConnect_FDA.indb 110 5/1/14 7:24 AM
License and Warranty
111
12
Limited License
WARNING
CAUTION – A RESTRICTED LICENSE LIMITS USE OF THE ACCU‑CHEK AVIVA CONNECT SYSTEM IN THE UNITED
STATES – READ CAREFULLY THE LIMITATIONS RECITED BELOW.
The ACCU‑CHEK Aviva Connect system (meter and test strips) and its use are protected by U.S. Patent Nos.
6,645,368 (expires 22‑December‑2017); 7,276,146 (expires 4‑October‑2022); 7,276,147 (expires
4‑October‑2022); 7,407,811 (expires 9‑May‑2020); 8,298,401 (expires 4‑October‑2022); 8,303,801 (expires
4‑October‑2022); 8,329,026 (expires 4‑October‑2022); 7,452,457 (expires 2‑May‑2026); 7,488,601 (expires
1‑February‑2026); 7,494,816 (expires 29‑December‑2019); 7,569,126 (expires 28‑December‑2026); and
7,604,721 (expires 12‑August‑2026). A license to use the ACCU‑CHEK Aviva Connect system is required until
the expiration of the last‑to‑expire patent listed above and is only granted when the ACCU‑CHEK Aviva
Connect meter is used with the ACCU‑CHEK Aviva Plus test strips.
ACCU‑CHEK Aviva Plus test strips are specifically manufactured for operation with the ACCU‑CHEK Aviva
Connect meter. Use of other test strips supplied by another manufacturer may prevent or impair the proper
function of the ACCU‑CHEK Aviva Connect system.
Using the ACCU‑CHEK Aviva Connect system indicates your acceptance of the restricted license to use the
ACCU‑CHEK Aviva Connect system only with ACCU‑CHEK Aviva Plus test strips. If you do not agree to the
terms and conditions of the restricted license, you may return, at the place of purchase, the unused
ACCU‑CHEK Aviva Connect system for a full refund. If you have any questions, please call the ACCU‑CHEK
Customer Care Service Center at 1‑800‑858‑8072.
Except where prohibited by statute, all warranties covering the ACCU‑CHEK Aviva Connect system are voided
by use of the ACCU‑CHEK Aviva Connect system with any test strips other than ACCU‑CHEK Aviva Plus test
strips.
WARNING
A RESTRICTED LICENSE LIMITS USE OF THE ACCU‑CHEK FASTCLIX SYSTEM (lancing device and lancet
drums). READ CAREFULLY THE LIMITATIONS RECITED BELOW.
The ACCU‑CHEK FastClix system (device and lancet drums) and its use are protected by U.S. Patent Nos
7,322,998 (expires 3‑March‑2020); and 7,785,338 (expires 5‑January‑2026). A license to use the
ACCU‑CHEK FastClix system is required until the expiration of the last‑to‑expire patent listed above and is
only granted when ACCU‑CHEK FastClix lancet drums are used with the ACCU‑CHEK FastClix device.
52195_AvivaConnect_FDA.indb 111 5/1/14 7:24 AM

License and Warranty
112
12
ACCU‑CHEK FastClix lancet drums are high precision components that are produced to the close tolerances
required for satisfactory operation with the ACCU‑CHEK FastClix device. Use of other lancet drums with the
ACCU‑CHEK FastClix device may prevent or impair proper function of the ACCU‑CHEK FastClix device.
Using the ACCU‑CHEK FastClix device indicates your acceptance of the restricted license to use the
ACCU‑CHEK FastClix device only with ACCU‑CHEK FastClix lancet drums. Further, if you have purchased an
ACCU‑CHEK FastClix device that includes this restricted license, then this restricted license applies
regardless of any additional oers found in ACCU‑CHEK FastClix device packages. If you do not agree to the
terms and conditions of the restricted license, you may return, at the place of purchase, the unused
ACCU‑CHEK FastClix device for a full refund. If you have any questions, contact the ACCU‑CHEK Customer
Care Services Center at 1‑800‑858‑8072.
Except where prohibited by statute, all warranties covering the ACCU‑CHEK FastClix device are voided by use
of the ACCU‑CHEK FastClix device with any lancet drums other than ACCU‑CHEK FastClix lancet drums.
Patent Information
U.S. Pat.: http://www.roche‑diagnostics.us/patents
52195_AvivaConnect_FDA.indb 112 5/1/14 7:24 AM

License and Warranty
113
12
52195_AvivaConnect_FDA.indb 113 5/1/14 7:24 AM

License and Warranty
114
12
52195_AvivaConnect_FDA.indb 114 5/1/14 7:24 AM

Index
115
A
averages 54
B
batteries, changing 95
battery drawer 95
battery type 95
blood glucose index 107
blood glucose results, unusual 37
blood glucose test, performing 31
button, functions 12
C
comfort dial 25
control results, out‑of‑range 24
control solution 19
control test, performing 20
D
data transfer
default 71
H
high blood glucose 38
hyperglycemia 38
hypoglycemia 38
I
icons 15
L
lancet drum, changing 28
lancet drum, inserting 26
lancing device, cleaning and disinfecting 91
lancing device, using 27
logbook 52
low blood glucose 38
M
maintenance, meter 95
meter, cleaning and disinfecting 88
P
pc reports
default 72
list report 83
standard day report 81
standard week report 82
trend 80
product limitations 103
product safety information 105
product specifications 103
S
safety information 3
settings, meter 39
beeper 42
language 49
last result 48
markers 45
target range 46
time/date 41
USB default 44
supplies 107
symbols 107
symptoms, hypoglycemia/hyperglycemia 38
Index
52195_AvivaConnect_FDA.indb 115 5/1/14 7:24 AM

Index
116
T
technical information 103
test strips 30
U
use by date 19, 30
V
view data, pc internet browser 74
view data in software 73
view pc reports 76
W
warranty 110
wireless communication 57
auto‑send 62
default device 61
delete paired device 67
flight mode 60
pair additional device 64
pairing 58
sync time 63
52195_AvivaConnect_FDA.indb 116 5/1/14 7:24 AM

Notes
117
52195_AvivaConnect_FDA.indb 117 5/1/14 7:24 AM

Notes
118
52195_AvivaConnect_FDA.indb 118 5/1/14 7:24 AM

52195_Book_Bridge_US_CVR.indb 3 4/28/14 2:25 PM
Roche Diagnostics
9115 Hague Road
Indianapolis, IN 46256
www.accu-chek.com
ACCU-CHEK, ACCU-CHEK AVIVA, ACCU-CHEK AVIVA CONNECT and FASTCLIX are trademarks of Roche.
The Bluetooth
®
word mark and logos are registered trademarks owned by Bluetooth SIG, Inc. and any use of such
marks by Roche is under license. Other trademarks and tradenames are those of their respective owners.
© 2014 Roche Diagnostics
52195-0514
Continua Certified signifies that this product complies with
applicable IEEE 11073-10417 standards and that it has
been tested and certified against the 2014 Continua Design
Guidelines.
52195_Book_Bridge_US_CVR.indb 4 4/28/14 2:25 PM